The creation of novel medications, treatments, and medical equipment that improve patient care and treatment results or outcomes is referred to as pharmaceutical innovation. Scientific technology breakthroughs and shifting regulatory environments have led to the innovation in pharmaceuticals which is essential for advancing in medicine development with a prime focus on enhancement of patient outcomes. Anticipating future improvements in the pharmaceutical industry requires an awareness of the drivers and obstacles of the complicated process that encompasses research and development, clinical trials, and dynamics of regulatory approvals. Collaboration amongst different stakeholders, such as researchers, doctors, regulatory agencies, and industry partners, has been a common trend in pharmaceutical innovation. This comprehensive approach guarantees patients receive novel medicines that are safe and effective. In addition, by encouraging transparent communication and knowledge exchange, the pharmaceutical business can surmount obstacles and expedite the release of novel treatments. The pharmaceutical sector has undergone a transformation because of the seamless technological breakthroughs. Technology has expedited the speed of innovation in a variety of fields, including robotic automation in medication manufacturing and better imaging tools for personalized treatment. Large volumes of data are being explored by researchers thanks to digital platforms and big data analytics, which is accelerating and improving the targeting of medication discovery. Furthermore, the identification and optimization of drug candidates is changing due to the integration of machine learning and artificial intelligence. In the process of developing new drugs, these technologies can evaluate intricate biological data and forecast the effectiveness of possible therapies, saving time and money. Pharmaceutical companies may expedite their research efforts and provide life-saving medications to patients more quickly by leveraging technology. [1]
Innovation continue to progress with new disease gene target & new frontiers in medicine
While evidencing these developments, which range from the creation of novel cancer immunotherapies that use the body's immune system to combat cancer cells (e.g. PD-L1 target) to the creation of gene therapies that identify and fix genetic mutations causing uncommon illnesses to filing the white space by focusing on the best drug analogue for go to market strategy such as PSCK9 inhibitors (like mAb, si-RNA, fusion proteins etc.) drug assessment against primary standard of care such as statins to hypercholesterolemia, have an enormous potential to enhance patient care and quality of life. Researchers and industry professionals are pushing the limits of what is possible in medicine and exploring new frontiers as the area of pharmaceutical innovation continues to progress. Amgen US after launching REPATHA (mAb targeting PCSK9), is now focusing on si-RNA based drug targeting Lp(a) outcome in cardiovascular diseases related to stroke while Novartis has also successfully launched PCSK9 inhibitor first-in-class si-RNA based LEQVIO to reduce LDL-C levels into the market. Healthcare companies are focusing on early diseases detection & developing vaccines for prevention against chronic diseases. [2,3]
It is clear that things are always changing in the field of pharmaceutical innovation. A primary factor propelling this development is the growing emphasis on biopharmaceuticals, encompassing biologics and biosimilars. These intricate compounds, which are sourced from living things, provide precision therapeutic alternatives for a range of illnesses, opening the door to more individualized and potent treatments. The field of drug development and discovery is advancing at a rate never seen before because to the introduction of artificial intelligence (AI) and machine learning. The drug development process can be greatly accelerated by using these technologies, which allow researchers to examine enormous volumes of data, find possible therapeutic candidates, and predict their efficacy with better precision. AI has the potential to be extremely important in patient care and clinical studies. Algorithms that use machine learning can evaluate patient data, pinpoint risk factors, and forecast how a treatment will work. This improves patient outcomes by empowering medical providers to make better decisions and offer individualized treatment solutions. AI-powered solutions can also help with remote healthcare delivery, medication adherence, and patient monitoring. Furthermore, by evaluating unstructured data from sources like social media, medical literature, and adverse event reports, artificial intelligence (AI) technologies like natural language processing (NLP) might improve pharmacovigilance efforts. Artificial intelligence (AI) can assist pharmaceutical businesses and regulatory agencies in ensuring the safety and efficacy of pharmaceuticals on the market by real-time alerting of potential safety problems or drug interactions. Pharma firms will keep an eye out for innovative technologies to improve internal operations and increase efficiency throughout the value chain, including generative AI, blockchain, immersives, and others. With their generative AI-designed molecule, EXS4318, from BMS and Exscientia, which entered Phase 1 trials in 11 months instead of the industry standard of four years, the two companies reduced the time it took to develop a medicine by 70%. Another area that is ready for disruption is clinical trials, as demonstrated by Novartis's investment in the AI startup YSEOP, which wants to automate and streamline these processes. [1,4]
Innovation with Biologics & biosimilar
By 2024, biologics should become increasingly prevalent. This is due to the fact that they provide more specialized and potent therapies for a range of illnesses. Biologics can target particular chemicals or cells involved in disease processes because they are derived from living organisms. As per the efficacy studies these are more effective and efficient than conventional small-molecule medications. More cutting-edge and potent biologic drugs are coming to the market as the technology continues to progress and research and development spending rises. According to The New York Times, we are living in a "golden age for medicine." Novel modalities such as mRNA, antibody drug-conjugates (ADCs), and microbiome-based therapies are emerging, while formerly revolutionary platforms and modalities like monoclonal antibodies (mAbs) have become conventional. Ozempic and Comirnaty, two of the top five selling medications today, are founded on cutting-edge modalities—peptide therapies and mRNA technology, respectively. This trend is likely to continue, if not pick up speed. A few significant developments followed, including the announcement in November 2020 of Genentech and Genesis Therapeutics' multi-target drug research cooperation, which used AI technologies to identify potential therapeutic options. In order to introduce Ogivri, a cancer medication that is a biosimilar to Herceptin, in the US market in 2019, Biocon and Mylan partnered. In January 2020, Bayer and the artificial intelligence drug discovery startup Exscientia partnered to research drugs related to oncology and cardiovascular illnesses.
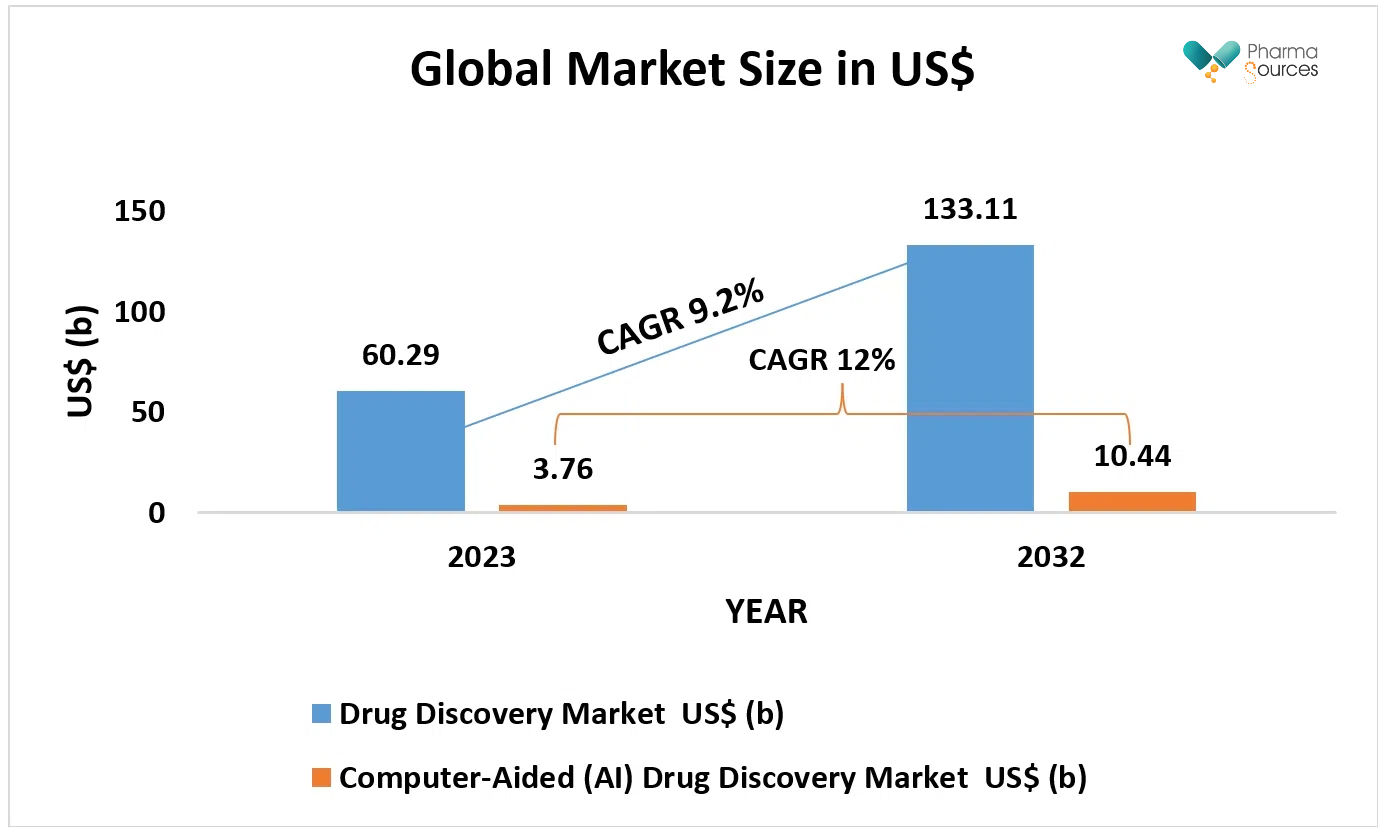
Figure: Graph showing forecast to global market size in US $ billions of drug discovery and computer-aided (AI) drug discovery with respective CAGR(%).[5,6]
In conclusion, there is domination of small molecule pharmaceuticals, the expanding use and adoption of biologics, the outsourcing of drug research and manufacture, the growing interest in personalized medicine with the advent of artificial intelligence, and the serious emphasis on emerging markets are some of the new trends that are generating a sense of expectation. Pharma firms also have to deal with issues including regulatory compliance, growing R&D costs, efficient supply chain management, obtaining intellectual property, managing the high prices associated with pharmaceuticals, and many other issues.
References:
1. Rubio, The Future of pharmaceutical innovation. Accessed on 02 September 2024.
URL : https://www.laboratoriosrubio.com/en/pharmaceutical-innovation-2/
2. Kciuk M, Yahya EB, Mohamed Ibrahim Mohamed M, Rashid S, Iqbal MO, Kontek R, Abdulsamad MA, Allaq AA. Recent Advances in Molecular Mechanisms of Cancer Immunotherapy. Cancers (Basel). 2023 May 11;15(10):2721. doi: 10.3390/cancers15102721. PMID: 37345057; PMCID: PMC10216302.
3. AMGEN, 8 Things to Know About Lipoprotein(a). Post date : Feb. 02, 2023; Accessed on Sep. 02, 2024 URL: https://www.amgen.com/stories/2023/02/8-things-to-know-about-lipoproteina
4. Bill Coyle and Karishma Trikha A coming (r)evolution? Pharma industry outlook, trends and strategies for 2024. Post date: Feb.13, 2024, Accessed on: Sep.02, 2024
URL: https://www.zs.com/insights/trends-shaping-pharmaceutical-landscape-2024-and-beyond
5. Precedence research, Drug Discovery Market Size, Share, and Trends 2024 to 2034. Last updated : June,2023; Accessed on : Sep.02, 2024. URL: https://www.precedenceresearch.com/drug-discovery-market
6. Precedence research, Computer-Aided Drug Discovery Market Size, Share, and Trends 2024 to 2034. Last updated : June,2023; Accessed on : Sep.02, 2024.
URL: https://www.precedenceresearch.com/computer-aided-drug-discovery-market





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

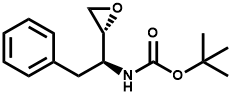
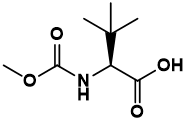




















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








