203 Spuer/PharmaSourcesAugust 07, 2023
Tag: Ten-billion market , CAR-T therapy , BCMA , Solid Tumor
Recently, Ikiorensai Injection, a BCMA targeting CAR-T product jointly developed by IASO Bio and Innovent, was approved for marketing in China for treating the adult patients with recurrent or refractory MM. It is the third CAR-T product approved in China and the ninth CAR-T product approved in the world.
To date, it has been nearly 6 years for global CAR-T therapy since Kymriah was approved in 2017. In terms of sales volume, the global CAR-T market has increased from US$10 million in 2017 to nearly US$2.7 billion in 2022.
Gilead has become the largest winner. Yescarta's sales volume reached US$1.16 billion last year, becoming the first famous CAR-T product. And there have been 3 kinds of CAR-T product in China in two years since Fosun Kite's Yescarta was approved in 2021. Within two years of approval, the annual revenue of JW Therapeutics' Beinuoda was CNY 146 million in 2022, with a year-on-year growth of 373.1%. Although the sky-high price of CNY 1.2 million a needle has frequently returned to hot search, the commercialization of CAR-T in China still brings the market some expectations under the situation that domestic medical insurance payment is stretched.
However, it is a pity that although there have been great breakthroughs in CAR-T therapy in several indications in the field of hematologic tumor and there have been famous products in five years, we all know that the prevalence rate of hematologic tumor is not high, which the annual increase of patients with hematologic tumor in the world accounts for less than 10% of all new tumor patients. In other words, there is imagination space left for the market, but not big.
So where is the wide market space? The answer is the field of solid tumors. The solid tumor is one of the two most deeply developed fields in CAR-T therapy, thus it is extremely urgent to overcome solid tumor in CAR-T therapy, considering domestic market access policy and current biomedical capital environment. Excellent clinical data on hematologic tumor also bring expectations to patients. In recent years, it is time to turn over the mountain of solid tumor for CAR-T.
According to the data, CAR-T exploration on solid tumor is still in the early stage. By the end of last year, there were 249 clinical trials registered in NIH in America, among which clinical research is basically in the early research stage (Phase I/II), and the distribution of targets is also extremely scattered, including GPC3, GD2, EGFR, MSLN. MUC-1, HER-2, PSMA and so on. Among them, the most CAR-T products are targeting MSLN and GPC3, which account for more than 20% of all clinical trials.
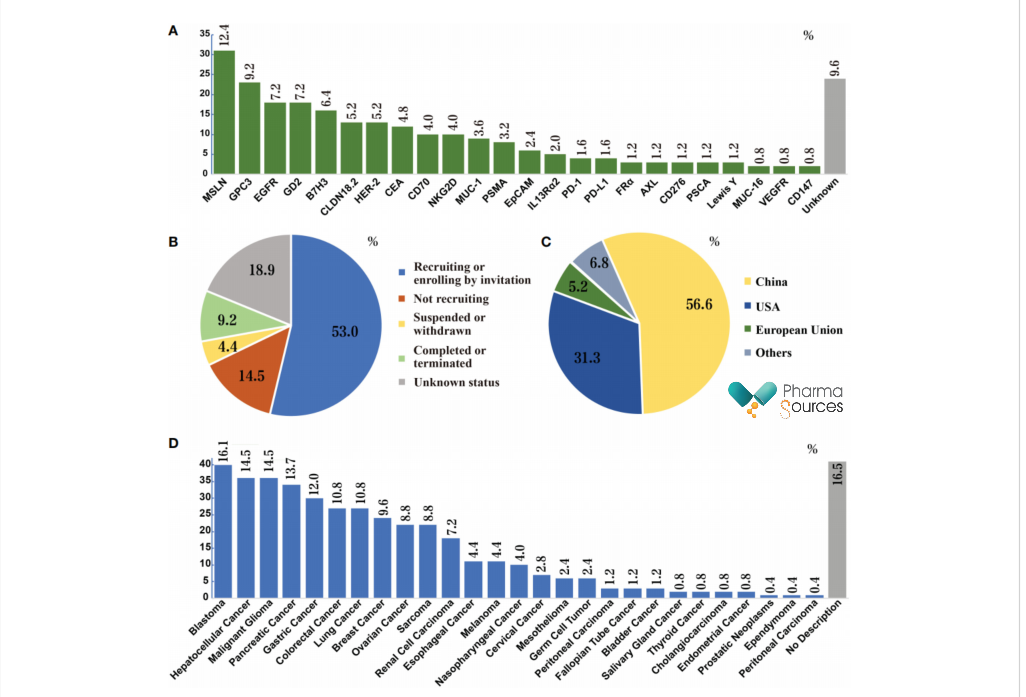
From the figure, it is not difficult to find that China is currently the most active country which explores CAR-T on solid tumors, with clinical trials exceeding the general proportion. For the indications, at present, the most exploration of CAR-T in solid tumors is blastoma (16.1%), hepatocellular carcinoma (14.5%) and malignant glioma (14.5%), while the exploration in major cancer types is slightly conservative: gastric cancer (12.0%) and lung cancer (10.8%).
Take MSLN as an example, it is a differentiation antigen existing in normal mesothelial cells, with no clear biological function in normal tissues, but the current evidence shows that MSLN has potential significance in tumor cell adhesion, progress, proliferation, survival and chemotherapy resistance. MSLN is overexpressed in many solid tumors, and CAR T cells targeting MSLN have been taken clinical researches in mesothelioma, epithelial ovarian cancer, PDAC, lung cancer, uterine cancer, triple negative breast cancer (TNBC), gastric cancer (GC), colorectal cancer, esophageal cancer, hepatocellular carcinoma (HCC) and neuroendocrine tumor/Merkel cell carcinoma.
As far as clinical design is concerned, many Phase I and II clinical trials have been taken at present. Monotherapy or combination therapy with other therapies (including ICIs and standardized drug therapy) is taken as the main treatment, which is in line with the mainstream choice of immunotherapy at present. In addition, CRISPR-Cas9 technology has also been used to genetically modify lentivirus-mediated anti-MSLN CAR T cells. Combining gene editing technology with CAR-T to promote the development of solid tumor indications, some early clinical data have been obtained at present, with good safety but poor validity data, which is also a common problem encountered in the exploration of CAR-T products in solid tumor indications.
Compared with the good clinical data obtained in BCMA CAR-T in multiple myeloma and CD19 CAR-T in B-cell lymphoma, in the first generation CAR-T therapy targeting multiple antigens (CAIX), CD171, FR-α, GD2, HER2, MSLN, EGFRvIII or VEGF-R2, there was no satisfactory result due to limited activity and repeated toxicity.
Different from hematological malignant tumors, microenvironment prevents the full activation and persistence of T cells in solid tumors. Firstly, a small amount of infiltration of T cells will be caused by the TME with hypoxia, low vascularization and rich extracellular matrix, and specific recognition will be weaken due to the loss of tumor antigen. In addition to the challenge of transporting CAR-T cells to the tumor site, the heterogeneity and poor immunogenicity of tumor cells are important problems to be solved. The followings are the challenges and current development strategies of CAR-T therapy in solid tumor development summarized by the author after referring to relevant literatures.
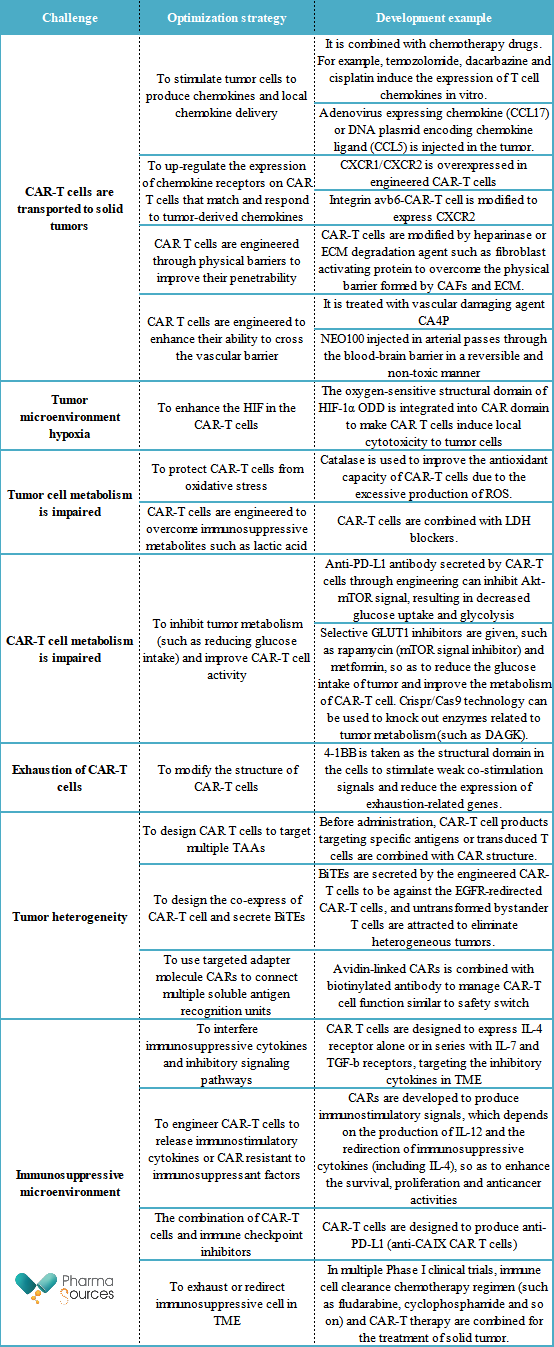
We can conclude from the above table that in order to overcome the difficulty of solid tumor, researchers are trying to improve CAR-T cell therapy by adjusting the design of CAR-T cells to make it a stronger killing ability and a better adaptability. For example, to combine the CAR-T cells and other therapies (such as antibody drug) can improve therapeutic effect in further. On the other hand, to optimize the orientation of CAR-T cells can make it identify and attack solid tumor cells more accurately.
In the future research, the primary task of CAR-T cells in the treatment of solid tumors is still to obtain more accurate and specific targets. As mentioned above, although the targets for solid tumors are scattered, the number is not large. Taking NSCLC as an example, at present most clinical researches continue to focus on common targets such as EGFR, HER2 and their mutations. And the new target may bring a breakthrough in solid tumor recognition for CAR-T cell therapy. What's more, it will further strengthen the target recognition specificity of CAR-T cells and avoid the occurrence of tumor off-target effect by improving the affinity of extracellular ScFv fragment of CAR and constructing the controllable CAR switch.
And cells are divided into different subtypes with the development of sequencing technology and bioinformatics, which provides more information for the structural design of CAR-T cells, and makes us better understand the signaling pathways between T cells and other TME cell components, as well as the intracellular cascade reactions related to the activation and exhaustion of CAR-T cells, which will make CAR-T cell therapy more successful in the treatment of solid tumors. What's more, it may make a breakthrough to study immune cells other than T cells.
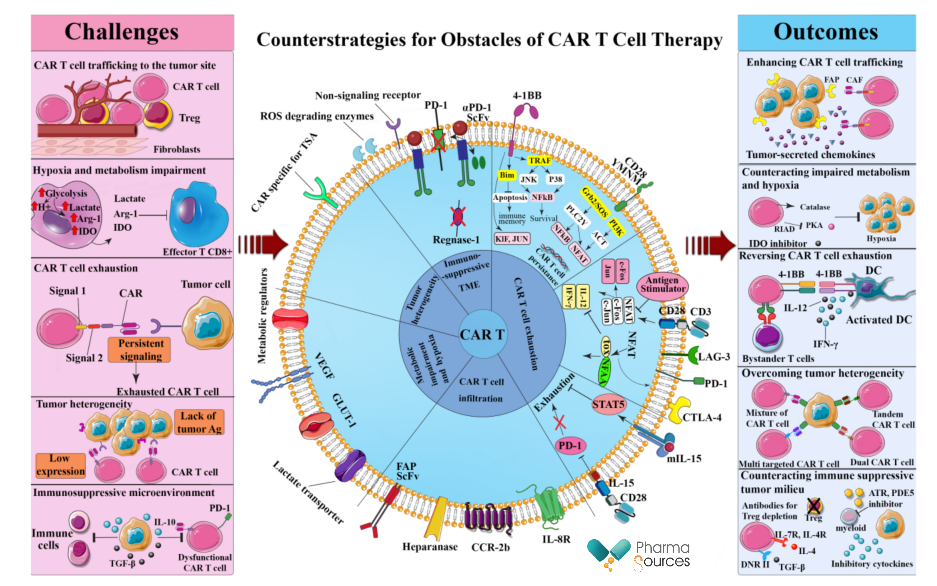
This paper does not mention the common problems of CAR-T therapy: clinical safety/effectiveness data, manufacturing costs, commercialization. Under the background that the global capital market is cold at present, the prospect of CAR-T has also encountered more uncertainties. As the most active area developing CAR-T in the world, China's enterprises are likely to succeed in the solid tumor field. With LEGN.US's product ahead, we believe that there must be domestic enterprises that can succeed in the next few years.
The current landscape of CAR T-cell therapy for solid tumors: Mechanisms, research progress, challenges, and counterstrategies,Front. Immunol. 14:1113882. doi: 10.3389/fimmu.2023.1113882
Bright future or blind alley? CART cell therapy for solid tumors,Front. Immunol. 14:1045024. doi: 10.3389/fimmu.2023.1045024
From bench to bedside: the history and progress of CAR T cell therapy,Front. Immunol. 14:1188049.doi: 10.3389/fimmu.2023.1188049
Combining chemotherapy with CAR-T cell therapy in treating solid tumors,Front. Immunol. 14:1140541.doi: 10.3389/fimmu.2023.1140541


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


