Nurah EkhlaqueApril 25, 2025
Nicotinamide adenine dinucleotide (NAD⁺) is a vital coenzyme that supports cellular energy metabolism and overall physiological function. As individuals age, NAD⁺ levels naturally diminish, a process associated with the onset of various age-related health conditions. To address this, NAD⁺ precursors like nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) are being explored for therapeutic use. These compounds show promise in treating neurodegenerative and metabolic conditions, prompting increased pharmaceutical interest.¹ In 2024, the global market for NAD⁺ products was estimated at around USD 494 million, with forecasts suggesting it could rise to USD 873.4 million by 2031, reflecting an expected compound annual growth rate of 14%.
This article highlights key applications, clinical trial developments, and regulatory classifications of NAD⁺ and its derivatives across major global markets.
Declining NAD⁺ levels have been linked to brain aging and neurodegenerative disorders like Alzheimer’s and Parkinson’s. Boosting NAD⁺ may enhance mitochondrial function, support DNA repair, and activate neuroprotective pathways such as sirtuins.²
Several NAD⁺ precursors are under investigation. While high-dose nicotinamide showed limited results in Alzheimer’s trials, newer agents like nicotinamide riboside (NR) have demonstrated promising early outcomes in Parkinson’s. A Phase 1 trial (NADPARK) showed NR raised brain NAD⁺ levels and reduced neuroinflammation. A more extensive Phase 2 clinical trial, known as NOPARK, is currently in progress.
● In Alzheimer’s research, NMN-based therapies such as MIB-626 have shown the ability to cross the blood-brain barrier and elevate NAD⁺ in cerebrospinal fluid. Follow-up studies will evaluate clinical benefits on cognition and disease biomarkers.
● Although no NAD⁺ therapy has yet achieved clinical approval for neurodegeneration, growing evidence supports its potential as a disease-modifying approach.
NAD⁺ plays a vital role in energy metabolism, insulin sensitivity, and mitochondrial function.Reduced levels of NAD⁺ have been associated with metabolic disorders such as type 2 diabetes, non-alcoholic fatty liver disease, and mitochondrial dysfunction.
Clinical studies show that NAD⁺ precursors like NMN and nicotinamide can improve insulin sensitivity, reduce liver fat, and enhance metabolic profiles. In rare mitochondrial disorders, niacin supplementation has successfully restored NAD⁺ levels and improved muscle performance.
While results vary, ongoing trials suggest NAD⁺ boosters may help manage metabolic conditions and support mitochondrial health, especially in aging or insulin-resistant populations.³
Declining NAD⁺ levels are linked to aging-related issues like reduced energy, impaired DNA repair, and muscle weakness. NAD⁺ boosters such as NMN and NR are being studied for their potential to support longevity by improving mitochondrial function and reducing cellular aging.³
Clinical trials show these supplements can safely raise NAD⁺ levels in older adults. Early findings suggest benefits like improved muscle endurance, reduced fatigue, and better cardiovascular function.⁴ While “anti-aging” isn’t a recognized medical indication, NAD⁺ repletion is emerging as a promising strategy to target age-related decline.
The classification of NAD⁺ and its precursors, including NR (nicotinamide riboside), NMN (nicotinamide mononucleotide), and NADH, differs considerably across countries. In certain regions, these compounds are permitted for use in dietary supplements or functional foods, whereas in others, they are regulated as novel ingredients or investigational substances.
In the U.S., NR is legally sold as a dietary supplement and has been acknowledged under the New Dietary Ingredient (NDI) notification process by the FDA. NMN, however, was excluded from supplement status in 2022 due to prior classification as an investigational new drug. Although this sparked industry backlash and legal pushback, NMN sales currently remain in regulatory limbo pending further FDA action. NADH is available as a supplement but has no FDA-approved drug use.⁵
Nicotinamide riboside (NR) is approved as a Novel Food in the European Union and is permitted for use in dietary supplements for adults, subject to defined dosage limits and labeling requirements. In contrast, nicotinamide mononucleotide (NMN) has not been authorized and remains prohibited until a formal safety assessment is completed by the European Food Safety Authority (EFSA). Neither NAD⁺ nor NADH is currently listed in the EU's approved novel food catalog, and their use in food products would likely require prior authorization.
China (SAMR/NMPA)
China has banned the sale of NMN and NR in foods and supplements, citing insufficient safety data. Imports via cross-border e-commerce were blocked in 2024. These compounds are not classified as drugs but are considered unapproved chemical ingredients unless formally registered. Currently, NMN is restricted to use in cosmetic applications and is not permitted for consumption.⁶
Japan (MHLW)
Japan is currently the most favorable market for NAD⁺ precursors. NMN has been approved as a food ingredient since 2020 and is widely sold as an anti-aging supplement. Products must comply with general food safety laws and may carry functional claims under Japan’s Foods with Function Claims (FFC) system. While NR and NADH lack explicit regulatory focus, they are generally accepted for supplement use.⁷
● Canada: NMN and NR are permitted as Natural Health Products (NHPs) with required licensing. NMN received a Health Canada monograph in 2024 for antioxidant use.
● Australia: NMN is not authorized for sale within the country but may be manufactured and distributed for export purposes. Personal import is permitted in small quantities.
● South Korea: NMN has not been approved for use in food products but is allowed for inclusion in cosmetic formulations. Regulatory approvals are pending for ingestible forms.
● UK & Others: Post Brexit, the UK continues to align with EU policy. NR is permitted, while NMN remains unauthorized and has been removed from major retailers such as Amazon. Singapore and Hong Kong allow NAD⁺ boosters, provided they are GMP compliant and do not carry medicinal claims.
The following table summarizes the regulatory status of NAD⁺ and its two major precursors (NMN and NR) in each region:
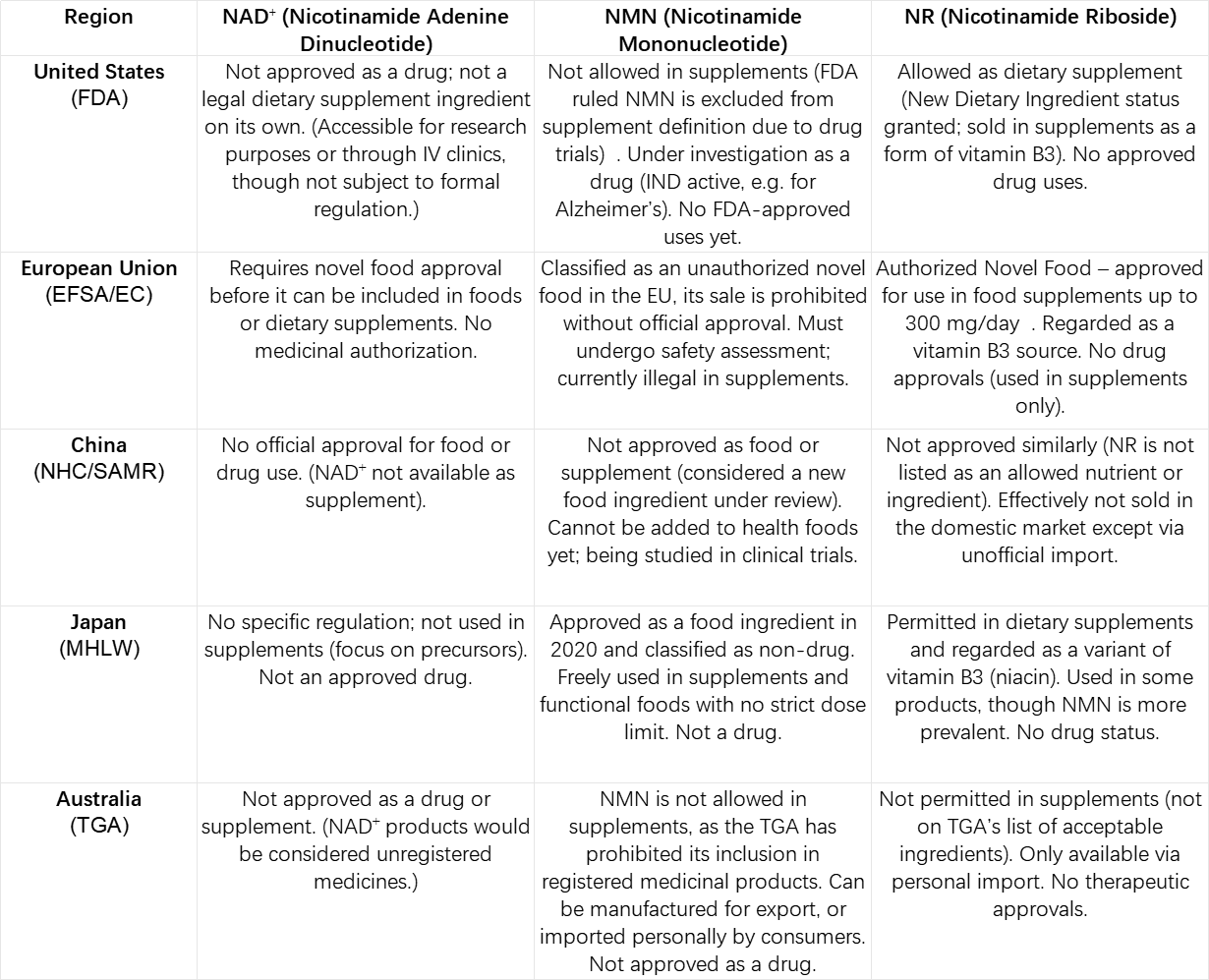
Table: Overview of how NAD⁺ and its key precursors (NMN and NR) are regulated across different countries. In all regions, no NAD⁺ booster is formally approved as a therapeutic drug to cure or treat diseases yet (all uses are either supplemental/nutritional or experimental). The US and Japan allow consumer use of at least one precursor (NR in US, both NMN/NR in Japan), whereas the EU, China, and Australia currently restrict NMN. NR is accepted in the US, EU, and Japan as a vitamin ingredient, but not in China/Australia. NAD⁺ itself is generally not directly marketed or needs special access. Regulatory definitions may change as more clinical data emerge.
Recent advances in longevity research have accelerated interest in NAD⁺ clinical use, particularly in addressing metabolic disorders, neurodegeneration, and age-related decline.
NAD⁺-based therapies have gained substantial attention in recent years, particularly within the fields of aging biology and chronic disease management. Scientific and commercial interest in molecules such as NMN and NR continues to grow, as researchers investigate their potential to support neurological, metabolic, cardiovascular, and muscular health.⁸
Companies are advancing proprietary formulations, including next-generation analogs like MIB-626, and developing combination products with polyphenols or other synergistic compounds to enhance therapeutic effects. The broad role of NAD⁺ in cellular function contributes to its potential as a multi-system therapeutic. Ongoing clinical trials in Parkinson’s disease, Alzheimer’s disease, heart failure, and age-related muscle decline reflect the wide-ranging applicability of these compounds. A single successful late-stage trial may significantly advance the status of NAD⁺ therapies in clinical medicine.⁹
NAD⁺ precursors have traditionally been sold as dietary supplements, particularly for general wellness and anti-aging support. However, increasing clinical research has moved these compounds toward pharmaceutical development. This transition has led to regulatory challenges.
For example, the United States Food and Drug Administration (FDA) reclassified NMN as excluded from the supplement category due to its prior status as an investigational drug. As a result of the decision, NMN was pulled from the U.S. supplement market, prompting legal disputes and opposition from the industry.
Some companies are now developing new formulations or delivery methods to secure intellectual property, as natural NAD⁺ precursors are not patentable. Future market structures may differentiate between over-the-counter supplements intended for general use and prescription-grade NAD⁺ therapies developed for specific diseases.
Barriers to Regulatory Approval
1. Clinical Effectiveness
Although NAD⁺ levels can be increased through supplementation, regulators require robust Phase 2 or 3 trial data showing tangible health benefits such as improved cognition or insulin sensitivity. Most current studies focus on biomarkers, not clinical outcomes.
2. Long Term Safety
NR and NMN appear safe short term (up to 12 months), but long term data is needed, especially for healthy individuals. A theoretical concern is whether prolonged NAD⁺ elevation could support tumor growth, though this has not been observed.
3. Labeling and Classification
Since aging is not classified as a disease, NAD⁺ products must target age related conditions such as frailty or neurodegeneration. Labeling laws vary by region. Health claims are permitted in countries like Japan and Canada, whereas regulatory frameworks in the United States and European Union impose more stringent restrictions. Harmonized global guidelines are needed.
Regulatory Conflict in the United States
The FDA’s drug preclusion ruling on NMN limited its supplement status and led to legal challenges. Possible compromises may follow the model used for compounds like NAC, balancing pharmaceutical interests with consumer access.
Global Disparities
Regulatory approaches differ significantly, with NMN approved in Japan and Canada but limited or restricted in other regions.Greater international coordination, including shared data and joint approvals, may help simplify future regulatory pathways.
NAD⁺ modulation holds strong potential in biotechnology and preventive medicine, with ongoing studies exploring its role in age-related and metabolic conditions.
If upcoming clinical trials confirm health benefits, NAD⁺ precursors may gain recognition as therapeutic agents. Until then, their use will vary across regions.The future of these compounds, whether as approved treatments or wellness supplements, depends on emerging research and regulatory decisions.
1. Radenkovic, Dina, et al. “Clinical Evidence for Targeting NAD Therapeutically.” Pharmaceuticals, vol. 13, no. 9, Sept. 2020, p. 247. PubMed Central, https://doi.org/10.3390/ph13090247.
2. Pirinen, Eija, et al. “Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy.” Cell Metabolism, vol. 31, no. 6, June 2020, pp. 1078-1090.e5. PubMed, https://doi.org/10.1016/j.cmet.2020.04.008.
3. Yoshino, Mihoko, et al. “Nicotinamide Mononucleotide Increases Muscle Insulin Sensitivity in Prediabetic Women.” Science, vol. 372, no. 6547, June 2021, pp. 1224–29. DOI.org (Crossref), https://doi.org/10.1126/science.abe9985.
4. Song, Qin, et al. “The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: An Update.” Advances in Nutrition, vol. 14, no. 6, Aug. 2023, pp. 1416–35. PubMed Central, https://doi.org/10.1016/j.advnut.2023.08.008.
5. FDA Halts NMN Supplement Approval, Citing Pharmaceutical Potential. https://www.nmn.com/news/fda-bans-labeling-nmn-as-a-supplement. Accessed 19 Apr. 2025.
6. “Daily News on Nutraceuticals, Functional Foods, Health Food and Ingredients in Asia and Oceania.” NutraIngredients-Asia.Com, 18 Apr. 2025, https://www.nutraingredients-asia.com.
7. Availability of NMN in Food Across the Major Countries/Regions_Antion Consulting_Antion Singapore Consulting Pte. Ltd. https://www.antion.net/En/Blog/view/id/q4ihjHbd8LuFag8YBYmxJAO0O0OO0O0O.html?utm_source. Accessed 21 Apr. 2025.
8. Rajman, Luis, et al. “Therapeutic Potential of NAD-Boosting Molecules: The in Vivo Evidence.” Cell Metabolism, vol. 27, no. 3, Mar. 2018, pp. 529–47. PubMed Central, https://doi.org/10.1016/j.cmet.2018.02.011.
9. Poljšak, Borut, et al. “Current Uncertainties and Future Challenges Regarding NAD+ Boosting Strategies.” Antioxidants, vol. 11, no. 9, Aug. 2022, p. 1637. PubMed Central, https://doi.org/10.3390/antiox11091637.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


