Nurah EkhlaqueApril 07, 2025
Tag: Cancer immunotherapy , Cancer vaccines , Neoantigens
Cancer immunotherapy has rapidly advanced in recent years, offering powerful ways to help the body’s immune system detect and destroy cancer cells. One of the most important breakthroughs has been the development of immune checkpoint inhibitors, which block proteins like PD-1, PD-L1, and CTLA-4 to restore immune response against tumors. These therapies have already improved survival outcomes across various cancer types. Now attention is shifting to a new generation of treatment called personalized cancer vaccines. These vaccines are developed based on the unique genetic profile of a patient’s tumor and are designed to target neoantigens, which are mutated proteins found only on cancer cells. Unlike traditional tumor markers, neoantigens offer high specificity, helping the immune system attack cancer more precisely while sparing healthy tissue.
By focusing on neoantigens, personalized vaccines offer key benefits such as strong and precise T-cell activation, fewer side effects, and better alignment with the patient’s immune system through major histocompatibility complex (MHC) matching. Early-phase clinical trials have shown encouraging safety and efficacy results.¹ With mRNA platforms enabling faster development and customization, neoantigen-based vaccines are emerging as a powerful approach in precision oncology.
Neoantigens are unique antigens produced by cancer cells due to genetic mutations. When a tumor’s DNA is altered through events like point mutations, insertions, deletions, or chromosomal changes, it can generate abnormal proteins. These proteins are broken down into peptide fragments and displayed on the surface of tumor cells. Since the immune system has never encountered these fragments before, it identifies them as foreign.
Because neoantigens originate from cancer-specific mutations and are absent from healthy tissue, they are ideal targets for immunotherapy. However, only a subset of mutations creates peptides that can bind to a patient's HLA molecules and activate T cells. These are the neoantigens of therapeutic interest. Identifying these enables scientists to design vaccines that train the immune system to recognize and attack cancer cells.
Countries worldwide are making significant progress in developing neoantigen-based cancer therapies. In China, firms like Genetron Health and GeneCast are targeting lung and liver tumors with personalized approaches. Moderna and Gritstone Bio in the U.S. are conducting trials for melanoma and glioblastoma. Meanwhile, Germany’s BioNTech is pioneering mRNA technologies with encouraging outcomes.¹ Together, these innovations reflect a strong international push toward precision cancer treatment.
Neoantigens stem from the genetic instability characteristic of tumors. As cancer grows, its DNA accumulates random mutations, some driven by environmental factors and others by internal errors. While many of these mutations are harmless passengers, a few act as drivers that fuel cancer progression. Both types can create neoantigens.
A single base change in DNA can alter a protein's amino acid sequence, resulting in a new peptide that the immune system perceives as a threat. Cancers with high mutation burdens, such as melanoma and lung cancer, often produce more neoantigens and respond better to immunotherapy. Additional sources of neoantigens include gene fusions, faulty RNA splicing, or mistranslated proteins. Each tumor's neoantigen profile is unique and shaped by its mutation landscape.
Neoantigens fall into two categories: private and shared. Private neoantigens are specific to an individual’s tumor, formed by unique mutations. These require fully personalized vaccine development. In contrast, shared neoantigens arise from common mutations in cancer driver genes like KRAS, TP53, or BRAF. These could potentially be used to create broader, non-personalized vaccines for groups of patients who share the same mutation.²
Although truly shared neoantigens are rare due to differences in how patients present peptides via their HLA types, they offer exciting potential. A shared neoantigen could allow for the development of off-the-shelf cancer vaccines, reducing costs and accelerating treatment. While most current approaches still rely on personalized vaccines, future strategies may include a hybrid model using both private and shared neoantigen targets.
Messenger RNA (mRNA) technology is transforming cancer immunotherapy by offering a highly adaptable and rapid vaccine platform. Unlike traditional vaccines that deliver proteins or peptides directly, mRNA vaccines provide genetic instructions that enable the body’s own cells to produce the target antigen.
For cancer vaccines, the mRNA is engineered to encode neoantigen peptides, which are unique markers derived from tumor mutations.This mRNA is encapsulated in lipid nanoparticles and injected into the patient. Once inside the body, cells absorb the mRNA and produce the encoded proteins. Dendritic cells, which are central to initiating immune responses, take up the mRNA, synthesize the neoantigen protein, and present fragments of it to T cells in the lymph nodes. This process effectively “trains” the immune system to recognize and eliminate tumor cells that display the same neoantigen.³
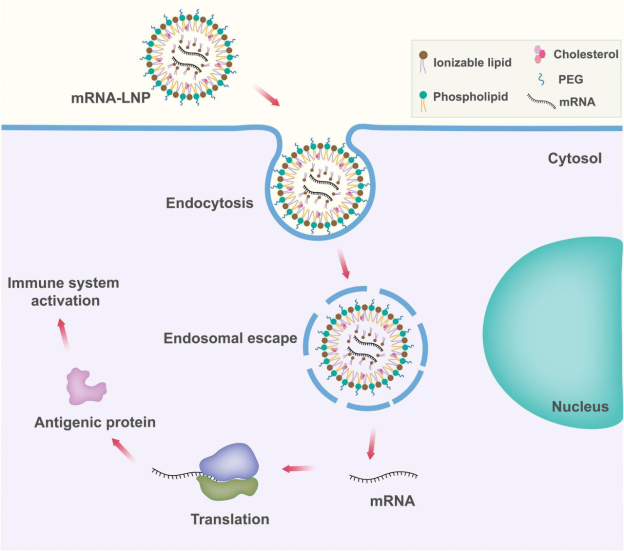
Figure X. Mechanism of mRNA-LNP vaccine delivery and antigen presentation. Adapted from Xu et al., 2022, Frontiers in Immunology (CC BY 4.0).
mRNA cancer vaccines offer several advantages. They are fast to design and manufacture. Once a patient’s tumor is sequenced and the neoantigens are identified, a tailored mRNA vaccine can be created within weeks. This rapid turnaround is possible because mRNA production is cell-free and adaptable. The same manufacturing system can be used across various cancers by changing the mRNA sequence.
mRNA vaccines are also inherently safe. They do not integrate into the patient’s genome and degrade naturally after delivering their message. Unlike protein-based vaccines, mRNA vaccines bypass the complex protein production process, reducing contamination risk and lowering production costs.⁴
Another benefit is their strong immune activation. mRNA vaccines can stimulate both T cell and antibody responses and can be enhanced with adjuvants when needed. The global success of mRNA COVID-19 vaccines has further proven their safety, scalability, and immunogenicity. Many of the companies behind these vaccines, such as BioNTech and Moderna, originally developed mRNA platforms for cancer treatment. Their pandemic experience is now accelerating innovation in oncology, with increased research investment and clinical trials aimed at creating personalized cancer vaccines.⁵
Personalized neoantigen vaccines have shown promising results in early trials. In a 2022 Phase II trial, Moderna and Merck’s mRNA vaccine (mRNA-4157/V940) combined with Keytruda reduced melanoma recurrence or death by up to 50%, leading to FDA Breakthrough Therapy status and a Phase III trial. A 2023 Phase I study in pancreatic cancer found that half of patients responded to BioNTech’s vaccine, with responders showing long-term T-cell immunity and extended cancer-free survival.⁶
Similar early-stage trials in glioblastoma, colorectal, kidney, and lung cancers report safety and immune activation. Institutions like Dana-Farber and MSK, alongside biotech firms such as Gritstone Bio and CureVac, are expanding research. Moderna’s new manufacturing site underscores the field’s momentum.
Though most data come from Phase I/II trials, these vaccines appear capable of generating lasting immune responses and improving outcomes, especially with standard treatments. Ongoing studies will help define their full clinical impact. The growing clinical success of personalized vaccines highlights the evolving role of cancer immunotherapy in delivering tailored treatments with durable outcomes.
Personalized neoantigen cancer vaccines offer a revolutionary approach to oncology, targeting unique tumor mutations to stimulate precise immune responses. Despite promising results, several challenges hinder their widespread clinical adoption.
● Neoantigen Identification: Identifying effective neoantigens is complex and time-sensitive. It requires tumor biopsy, extensive DNA/RNA sequencing, and advanced bioinformatics to predict which mutations will generate immune responses. Errors in this process can render the vaccine ineffective. Although machine learning tools are improving prediction accuracy, the process remains resource-intensive and technically demanding.
● Manufacturing Hurdles: Each vaccine must be custom-made, involving the synthesis of patient-specific mRNA and formulation with lipid nanoparticles. This process takes weeks and demands strict quality control, making scalability and cost a major issue. Companies like Moderna are building facilities to streamline production, but widespread implementation remains limited.
● Regulatory and Approval Complexities: Regulators face challenges approving individualized treatments. Agencies are exploring models to approve manufacturing platforms rather than individual batches, but safety and efficacy must still be validated for each patient. Most efforts remain in early-phase trials due to these constraints.
● Logistics and Cost Barriers: From sequencing to delivery, the workflow requires tight coordination among labs, manufacturers, and clinics—something only a few centers can manage. Costs remain high, limiting access to patients in well-funded health systems. Approaches such as shared neoantigens and centralized production could improve efficiency and reduce expenses.
● Ethical and Equity Issues: Genomic data privacy and equitable access are key concerns. Ensuring patient consent, safeguarding data, and making these therapies available beyond elite institutions are vital for ethical implementation.⁷
Neoantigen-based mRNA cancer vaccines represent a promising advancement in precision oncology, offering treatments tailored to each patient’s unique tumor profile. Although regulatory and manufacturing hurdles persist, continued progress in science and technology signals a future where personalized cancer vaccines become an integral and effective component of standard cancer care.
1. Li, Xiaoling, et al. ‘Neoantigen Cancer Vaccines: A New Star on the Horizon’. Cancer Biology & Medicine, vol. 21, no. 4, Apr. 2024, pp. 274–311. PubMed Central, https://doi.org/10.20892/j.issn.2095-3941.2023.0395.
2. Pearlman, Alexander H., et al. ‘Targeting Public Neoantigens for Cancer Immunotherapy’. Nature Cancer, vol. 2, no. 5, May 2021, pp. 487–97. www.nature.com, https://doi.org/10.1038/s43018-021-00210-y.
3. Vishweshwaraiah, Yashavantha L., and Nikolay V. Dokholyan. ‘mRNA Vaccines for Cancer Immunotherapy’. Frontiers in Immunology, vol. 13, Dec. 2022, p. 1029069. PubMed Central, https://doi.org/10.3389/fimmu.2022.1029069.
4. Sayour, Elias J., et al. ‘Cancer mRNA Vaccines: Clinical Advances and Future Opportunities’. Nature Reviews Clinical Oncology, vol. 21, no. 7, July 2024, pp. 489–500. www.nature.com, https://doi.org/10.1038/s41571-024-00902-1.
5. Park, Jung Woo, et al. ‘mRNA Vaccines for COVID-19: What, Why and How’. International Journal of Biological Sciences, vol. 17, no. 6, Apr. 2021, pp. 1446–60. PubMed Central, https://doi.org/10.7150/ijbs.59233.
6. Dolgin, Elie. ‘Personalized Cancer Vaccines Pass First Major Clinical Test’. Nature Reviews Drug Discovery, vol. 22, no. 8, July 2023, pp. 607–09. www.nature.com, https://doi.org/10.1038/d41573-023-00118-5.
7. Wu, Da-Wei, et al. ‘Personalized Neoantigen Cancer Vaccines: Current Progression, Challenges and a Bright Future’. Clinical and Experimental Medicine, vol. 24, no. 1, Sept. 2024, p. 229. Springer Link, https://doi.org/10.1007/s10238-024-01436-7.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


