Shruti TalashiNovember 21, 2024
Tag: Lifecycle , drug safety , QRM
At every stage of the product lifecycle, including research, pre-clinical, registration, clinical trials, processing, manufacturing equipment, instrument validation, quality maintenance, packaging, labeling, and more, pharmaceutical companies are subject to strict regulations and must maintain compliance with local governing bodies such as the FDA (Food & Drug Association), EMA (European Medical Agency), PMDA (Pharmaceuticals and Medical Devices Agency, Japan), China's National Medical Products Administration (NMPA), CDSCO (Central Drugs Standard Control Organization, India), SFDA (Saudi Food and Drug Authority), and more. The pharmaceutical industry relies heavily on companies adhering to the regulations' lifespan, which is the basis for FDA approvals. Promoting and upholding the guarantee of drug use is the aim of the regulation. Throughout the drug life cycle, drug prices fluctuate and sharply decline following loss of exclusivity (LOE), and maybe earlier as a result of growing competition and probable price or volume agreements. Promoting and upholding the guarantee of drug use is the aim of the regulation. All stages of the drug's lifespan are logically defined by these regulations. These regulations coherently define the lifecycle of the drug at all phases. A novel drug's development is a multi-stage, time-consuming, and intricate procedure. It starts with the discovery phase, during which compounds with therapeutic promise are found and possible drug targets are identified. The next step is preclinical testing, which assesses these compounds' effectiveness and safety in both lab and animal experiments. In order to evaluate safety, efficacy, and dose, the medication undergoes phase I, II, and III clinical trials using human participants if it shows promise. Regulatory approval from organizations such as the FDA is sought following the successful conclusion of clinical trials. Following approval, the medication is put on the market and subjected to post-market surveillance to ensure its effectiveness and safety in actual use.[1]
It takes skill and diligence to navigate the regulatory environment of pharmaceutical product life cycle management. Businesses can improve compliance, reduce risks, and speed regulatory procedures by proactively addressing issues and enlisting outside help. External support provides crucial direction and experience at every level, from post-market surveillance to regulatory compliance and reporting. Businesses may successfully negotiate complexity and guarantee the success and integrity of their goods throughout their life cycle with the help of the appropriate partners and a clear regulatory plan. Adverse event reporting is a crucial component of post-market surveillance and pharmacovigilance since businesses are required to notify regulatory bodies of adverse occurrences. The FDA's MedWatch program requires that significant adverse events be reported within 15 days.The European Medicines Agency's EudraVigilance is a centralized database for reporting adverse events in Europe. While the FDA and EMA mandate the production of Periodic Safety Update Reports (PSURs) at predetermined intervals (often annually), PSURs are regular submissions of safety reports. The FDA and EMA require Risk Management Plans (RMPs) for new medications and major post-approval adjustments. RMPs are documents that describe the risk management system. [2]
From product development to post-market surveillance, quality risk management (QRM) is essential in identifying and reducing possible threats to the quality of the final product. The systematic process of identifying, evaluating, controlling, and reviewing threats to the quality of pharmaceutical goods across the course of their lifecycle is known as QRM. This covers phases such as post-market surveillance, manufacture, distribution, and development. Pharmaceutical firms use Good Manufacturing Practices (GMP), Quality Control (QC), and Quality Assurance (QA) to guarantee the quality of their products. To improve quality control, contemporary technologies like data analytics, automated inspection, and artificial intelligence are being used in addition to conventional techniques. Because quality directly affects patient safety, efficacy, and regulatory compliance, it is of utmost importance in the pharmaceutical sector. It reduces risks, upholds public confidence, and guarantees that drugs work consistently. From development to post-market surveillance, QRM is essential at several points in the product lifecycle.[3]
Pharmaceutical product lifecycle management, or PLM, is the strategic management of a pharmaceutical product's lifecycle, including its conception, development, debut onto the market, growth, maturity, and decline. An efficient PLM can boost a company's ability to innovate, stay in compliance with regulations, optimize processes, and boost product profitability. Strict pharmaceutical regulatory compliance is essential to ensuring patient safety, regulatory compliance, and industry integrity. A strategy method for directing a pharmaceutical product from its conception to its removal from the market is called pharmaceutical product lifecycle management, or PLM. It includes a number of steps, starting with research and development, which involves creating compounds and identifying possible therapeutic targets. Clinical trials follow preclinical testing, which assesses safety and effectiveness in lab and animal experiments. Clinical trials in humans are conducted to examine safety, effectiveness, and dose after preclinical testing in lab and animal research. The medication is commercialized and introduced to the market after regulatory bodies have given their approval. The safety and effectiveness of the medication are continuously monitored thanks to post-market surveillance. The product's lifecycle may be prolonged as it develops by tactics like reformulation, additional indications, or new delivery systems. Successful global launches require a strong global regulatory strategy that includes regulatory information, filings, and compliance. Pharmaceutical businesses can guarantee the safety, effectiveness, and financial success of their goods by efficiently managing each step.[4]
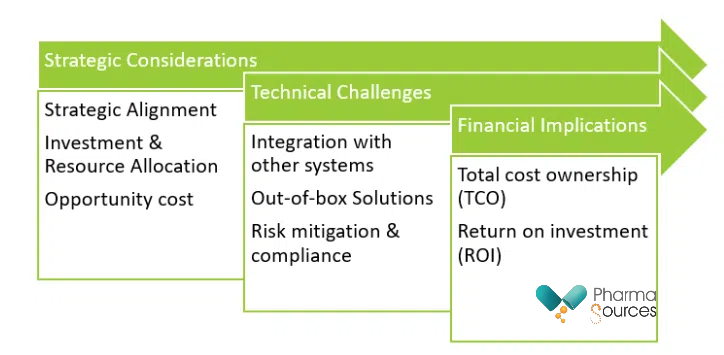
Figure is a flowchart to understand the multifaceted cost landscape of PLM with categorization of the factors into three broad groups
Although PLM has made great progress in streamlining product development procedures, there is still room to expand its influence. PLM can become a genuinely transformational technology by tackling issues like data visibility, integration with Industry 4.0 techniques, and wider organizational acceptance. Unlocking PLM's full potential and promoting innovation, efficiency, and sustainability in the years to come will require investing in innovative technologies like artificial intelligence (AI) and machine learning (ML) as well as cultivating a culture of data-driven decision-making. PLM technology is expected to undergo even more radical change in the future due to new developments and changing business requirements. PLM systems will be essential to the management and analysis of data from linked devices as the grows. This will allow for real-time performance monitoring, predictive maintenance, and improved product design. Furthermore, PLM capabilities will be further improved by integrating ML and AI, which will make automation, predictive analytics, and intelligent decision-making possible. Furthermore, as businesses concentrate on environmentally responsible design, production, and end-of-life management, sustainability will emerge as a major force in PLM. By monitoring the effects on the environment, maximizing the use of resources, and promoting the circular economy, PLM systems will assist sustainable practices. As cloud-based PLM systems become more popular, they will enable businesses to cut expenses, speed up innovation, and collaborate easily. Businesses may realize PLM's full potential and attain sustainable success in the digital era by adopting these technology innovations and strategic methods.[5]
A potent technique for cutting costs and managing expenditures during the medication research and commercialization process is product lifecycle management, or PLM. PLM helps businesses improve resource allocation and lower operating costs by expediting time-to-market, enhancing collaboration, and simplifying procedures. Better quality control is also made possible by PLM, which lowers the possibility of expensive product recalls and legal problems. Pharmaceutical firms can greatly improve their financial performance and keep a competitive edge by utilizing PLM and putting techniques like outsourcing, lean manufacturing, and supply chain optimization into practice. A pharmaceutical business overcame issues with data management, development schedules, and regulatory compliance by effectively implementing a PLM solution. The organization benefited greatly from centralizing product information, automating workflows, and improving communication. These included quicker time to market, better-quality products, and more stringent regulations. Improved product quality, quicker time to market, better regulatory compliance, higher productivity, and lower expenses were some of these. Combining PLM with cutting-edge technologies like AI and data analytics enabled the business to make data-driven decisions, allocate resources optimally, and eventually provide patients with high-quality products.[6]
To summarize as PLM is a critical process that ensures the safe, effective, and compliant development, manufacturing, and commercialization of drugs. By effectively managing each stage of a drug's lifecycle, from research and development to post-market surveillance, pharmaceutical companies can reduce costs, accelerate time-to-market, and improve patient outcomes. Strict regulatory compliance is a cornerstone of PLM. Adherence to regulations set forth by agencies like the FDA, EMA, PMDA, NMPA, CDSCO, and SFDA is essential to mitigate risks, protect public health, and maintain market access. Quality risk management (QRM) plays a vital role in PLM by identifying and addressing potential quality issues throughout the drug lifecycle. By implementing robust quality control and assurance measures, pharmaceutical companies can ensure product quality and consistency. As the pharmaceutical industry continues to evolve, PLM is becoming increasingly important. By leveraging advanced technologies such as AI, machine learning, and IoT, companies can further optimize their PLM processes, improve decision-making, and enhance overall efficiency. In conclusion, PLM is a strategic imperative for pharmaceutical companies. By effectively managing the entire drug lifecycle, companies can achieve sustainable growth, deliver innovative therapies, and ultimately improve patient care.
1. The Lifecycle of Drug Development, VIAL. Accessed on November 18, 2024 URL: https://vial.com/blog/articles/drug-development-lifecycle/?https://vial.com/blog/articles/drug-development-lifecycle/?utm_source=organic
2. Trial Expert, CREDEVO. Published on: May 25, 2024; Accessed on: November 18, 2024 URL: https://credevo.com/articles/2024/05/25/effective-regulatory-life-cycle-management-in-pharma-addressing-common-challenges/#:~:text=In%20pharmaceutical%20life%20cycle%20management,complexities%20of%20product%20development%20and
3. Bloglet, Compliance quest. Accessed on: November 18, 2024 URL: https://www.compliancequest.com/bloglet/quality-risk-management-in-pharmaceutical-industry/
4. Maven regulatory solutions, Navigating Pharmaceutical Product Lifecycle Management And Regulatory Compliance: Strategies For Success. Accessed on : November 18, 2024 URL: https://www.mavenrs.com/Navigating-Pharmaceutical-Product-Lifecycle-Management-and-Regulatory-Compliance-Strategies-for-Success
5. SAP, Supply Chain Management /Product Lifecycle Management. Accessed on : November 18, 2024 URL: https://www.sap.com/india/products/scm/plm-r-d-engineering/what-is-product-lifecycle-management.html
6. John McCurdy , The Benefits of Cloud-Based PLM Software. Published on: December 13, 2023; Accessed on : November 18, 2024 URL: https://www.aptean.com/en-US/insights/blog/cloud-based-plm-software-benefits#:~:text=Cloud-based%20PLM%20systems%20offer,and%20your%20organization%20just%20needs
boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


