Saher HaiderDecember 09, 2024
Tag: antibiotics , Pediatrics Formulations , APIs
Given children's unique physiological and pharmacokinetic characteristics, the development of pediatric antibiotic formulations requires stringent controls. Factors like physiological differences, dosage flexibility, palatability and compliance, excipient safety, and stability are critical “formulation”-related issues that must be addressed in pediatric antibiotics.
However, before tackling these formulation challenges, the foundation must be set, which revolves around sourcing safe and high-quality antibiotic APIs.
If you’re a professional responsible for procurement, quality assurance, or compliance, this article will give you a roadmap to sourcing reliable and safe antibiotic APIs tailored to pediatric needs.
In this article, we’ll provide you with a stepwise method for sourcing pediatric-appropriate antibiotic APIs that not only meet therapeutic efficacy standards but also align with stringent global regulatory guidelines.
So, without further ado, let’s get started!
The distinct physiological and pharmacokinetic characteristics of children make sourcing pediatric antibiotic APIs a complex and highly regulated task. Children’s metabolic rates, organ development stages, and body water content differ from adults', affecting how drugs are absorbed, distributed, and eliminated.
As a result, APIs in pediatric antibiotics must be meticulously designed for precise dosing and consistent efficacy. Even more important is that APIs must be highly pure to prevent unintended side effects.
Choosing the appropriate antibiotic API for pediatric use is a daunting task that involves several factors including:
Antibiotics for pediatric use must be effective against targeted pathogens and possess a favorable safety profile in children.
The API’s compatibility with chosen excipients is essential to avoid toxic impurities or degradation of products that could affect the medication’s safety or efficacy. For example, antibiotics that are unstable in certain formulations may need special processing techniques, such as nano-sizing or salt formation, to enhance bioavailability and stability for pediatric use.
Solubility and stability are important in determining the suitability of an API for pediatric formulations. For instance, poorly soluble drugs may require innovative approaches to improve their solubility and ultimately enhance their bioavailability in children.
Choosing the appropriate antibiotic API is vital, but the rigorous sourcing of high-quality APIs is just as important to developing safe pediatric antibiotic formulations. The following are the main factors to consider when sourcing high-quality APIs:
Antibiotic APIs for pediatric applications must meet stringent standards from the FDA, EMA, and ICH to ensure safe and effective usage in children. Adherence to these standards is pivotal to maintaining consistent quality in API production, lowering the risk of contamination, and keeping pediatric formulations safe for use.
The ICH Q7 guidelines for GMP in API mandate control over contamination, proper facility maintenance, and thorough personnel training—all of which are critical factors in pediatric safety. As procurement professionals, you can ensure that your potential suppliers comply with this guideline and produce high-quality APIs, with a rigorous focus on contamination control.
For pediatric-specific guidelines, the EMA’s Pediatric Regulation (EC No 1901/2006) and the U.S. Pediatric Research Equity Act (PREA) emphasize that antibiotics undergo comprehensive trials to assess safety and efficacy in children. These regulations require that all medicines with pediatric applications adhere to additional safety assessments.
Essential Certifications and Supplier Qualifications
The high-quality sourcing of antibiotic APIs requires suppliers to hold GMP certification to verify that they adhere to strict manufacturing standards. As procurement professionals, the following are examples of certifications to look out for during supplier qualifications:
ISO 9001 certification for demonstrating the implementation of quality management systems, ensuring suppliers maintain consistent quality control standards across all operations
ICH Q7 certification for demonstrating adherence to GMP requirements related to maintaining contamination control, documentation, and quality management.
Certificates of Analysis (CoA). This certificate confirms that each API batch meets specified quality and purity standards,including assays, impurity levels, and microbial limits. Reviewing the CoAs confirms that the supplier’s APIs are free of contaminants that could compromise pediatric formulations.
Batch Records and Process Validation Reports. Batch records provide a complete history of manufacturing processes, including minor details of any changes in production processes and confirming the quality of each batch.
Stability Data and Storage Protocols. Unlike other pharmaceutical products, pediatric formulations often require specific stability conditions to minimize risks associated with product degradation and ensure that APIs maintain their quality throughout their shelf life.
Selecting suppliers with a strong reputation for producing high-quality antibiotic APIs is important. Reliable suppliers undergo regular inspections by regulatory authorities and maintain rigorous quality control processes so that every batch meets safety and purity standards. Procurement professionals who verify a supplier’s compliance history help them select and include only trusted partners in the supply chain.
When sourcing antibiotic APIs for pediatric products, a pharmaceutical company’s procurement and compliance professionals can ask their suppliers to provide detailed information on the production processes used for API manufacturing. It requires suppliers to be transparent regarding potential contaminants, allergens, or the presence of any processing additives to assess the API’s suitability and safety for pediatric applications.
Risk assessment and due diligence are foundational steps in evaluating the suitability and safety of APIs for pediatric formulations where patient safety requirements are more stringent. But before diving into the process of conducting risk assessment and due diligence, let us first understand the two terms:
Risk Assessment is the process of identifying, analyzing, and evaluating potential risks associated with a specific process or product. It includes examining factors such as purity, stability, and contamination risks that could impact the quality, safety, or efficacy of the API.
On the other hand, Due Diligence refers to the thorough investigation and evaluation of a supplier’s compliance, manufacturing practices, and track record. It is an ongoing process that involves verifying certifications, assessing regulatory compliance, and ensuring that suppliers maintain high standards in API production.
Together, risk assessment and due diligence create a proactive approach to mitigating potential safety and quality risks associated with sourcing APIs for pediatric use.
Procurement professionals should evaluate the following areas when conducting risk assessment and due diligence for antibiotic APIs:
Impurities in APIs can arise from residual solvents, degradation byproducts, or cross-contamination, which can lead to serious side effects. Establishing a clear understanding of the impurity profile of APIs helps identify any potential risks to pediatric safety.
Pediatric formulations may require unique stability profiles to suit different dosage forms, such as liquids, powders for reconstitution, and buccal or chewable forms, all of which are commonly used for younger patients. Stability assessments should include exposure to temperature fluctuations and humidity to verify the safety and efficacy of API across various conditions.
Manufacturing facilities that produce multiple products pose the risk of cross-contamination. Due to the increased vulnerability of pediatric patients, the API production environment should be controlled to prevent contamination from other substances. This risk can be minimized by ensuring that the supplier facilities maintain strict segregation and contamination control practices.
A compliance checklist for vendor evaluation serves as a practical reference for procurement professionals, quality control personnel, and regulatory personnel to verify that each supplier and API batch meets the high standards required for pediatric applications, reducing risks associated with contamination, substandard quality, and regulatory non-compliance.
Below is a checklist table that sums up the criteria for evaluating suppliers and their products in line with regulatory expectations and best practices:
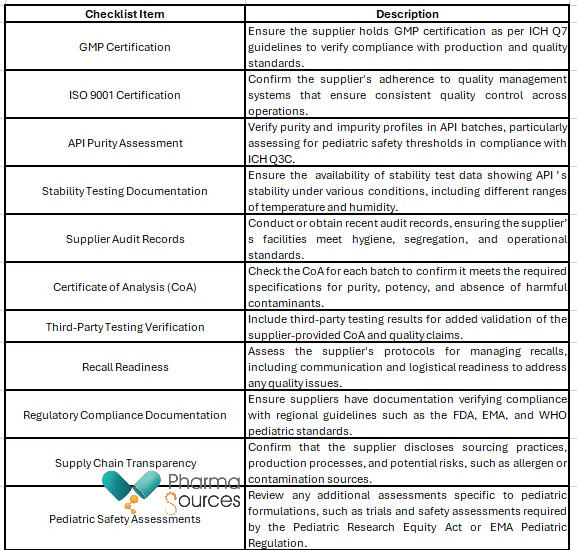
Sourcing high-quality APIs for pediatric applications requires a comprehensive approach to supplier evaluation, rigorous adherence to regulatory standards, and an emphasis on proactive risk management. For instance, in 2018, the FDA recalled multiple batches of valsartan, a common blood pressure medication, due to NDMA contamination in APIs sourced from overseas suppliers. While not a pediatric antibiotic, this case underlines the potential consequences of lapses in supplier oversight. A thorough compliance checklist could have flagged NDMA risks earlier, preventing exposure and safeguarding patient health. For pediatric antibiotics, following a similar structured approach through detailed checklists enables pharmaceutical companies to mitigate contamination risks, maintain regulatory compliance, delivering safe and effective treatments to pediatric patients
PharmD, Medical & Life Sciences Content Creator
Saher Binte Haider is a pharmacy graduate from Dow University of Health Sciences. She started her career as a Quality Management professional in the pharmaceutical industry where she developed a keen interest in good documentation practices, SOP creation, and content writing. She has 7+ years of experience in healthcare & life sciences content writing. Her key areas of expertise are healthcare, pharmaceuticals, health tech, and AI in healthcare.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


