Krebs QinOctober 18, 2024
Tag: PD-1 , Inhibitor , Oncology
In the field of oncology, Opdivo and Keytruda, as core immunotherapy agents for PD-1 inhibitors, have delivered significant survival benefits to patients with a variety of cancers. With its excellent efficacy and wide range of indications, Keytruda and Opdivo have occupied an extremely important position in the global oncology market. Their market performance not only solidifies their lofty position as a leader in immunotherapy, but also demonstrates the central role of PD-1 inhibitors in cancer treatment. In the second quarter of 2024, Keytruda and Opdivo achieved theie sales performance of USD7.27 billion and USD2.39 billion respectively, ranking first and third in oncology drugs (the second was Johnson & Johnson /Genmab's multiple myeloma drug Darzalex at USD2.88 billion). Figure 1 shows the 2023 sales volume and indications for the 10 types of PD-1 inhibitor oncology products approved by the FDA.
Comparison of Sales and Indications of PD-1 Inhibitor Approved by the FDA
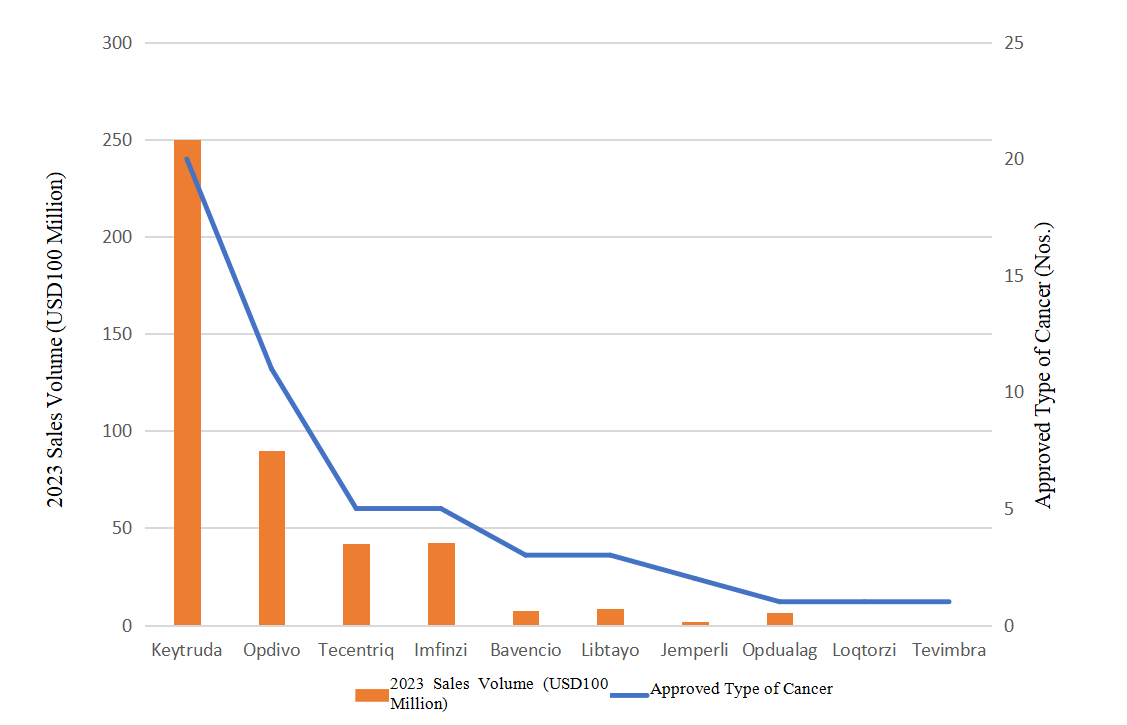
Figure 1. Comparison of Sales Volume and Approved Indications of PD-1 Inhibitor Approved by the FDA Oncology Drugs in 2023. (Note: Loqtorzi and Tevimbra were approved in 2022 and 2023 respectively, with no sales volume data for 2023)
Although PD-1 inhibitors are still an important pillar of tumor immunotherapy, with the continuous progress of scientific research, a series of new drugs are providing patients with more treatment options. These innovative therapies show unique mechanisms and potential to complement PD-1 inhibitors, opening new doors for cancer treatment and allowing us to see the possibility of a more diverse tumor treatment in the future.
Checkpoint inhibitors have revolutionized cancer treatment, but they still face powerless cases and patients. Some patients receive PD-1 inhibitor drugs, but the disease is still progressing. In terms of the gaps left behind by PD-1, Replimune believes that their cancer vaccine RP1 (vusolimogene oderparepvec) has the potential to become the go-to protocol for melanoma patients who have not responded to PD-1 inhibitors, and they have published impressive IGNYTE test data.
RP1 is an oncolytic immunotherapy based on HSV-1 (herpes simplex virus 1) gene modification. RP1 can selectively infect and kill cancer cells without serious damage to normal cells. After infecting cancer cells, RP1 replicates and destroys these cells, releasing tumor antigens. RP1 can not only kill cancer cells directly, but also activates the patient's immune system through the release of tumor antigens, resulting in a systemic immune response to the tumor. This immune activation works like a vaccine, helping the body recognize and attack other cancer cell. In addition, RP1 also expresses GM-CSF (cell-macrophage colony-stimulating factor) and fusion proteins. GM-CSF can stimulate antitumor cells in the immune system (such as dendritic cells and macrophages) to enhance their ability to recognize and kill cancer cells. By enhancing the display of tumor antigens, GM-CSF makes cancer cell death have more immunogenicity, helping to stimulate a stronger immune response. GM-CSF's expression can also enhance the local and systemic immune response, prompting the immune system to recognize and attack distant tumor lesions. Moreover, the GALV-GP R protein encoded by RP1 enhances the tumor killing capacity of the virus and increases the cell death of immunogenicity. In other words, RP1 not only uses the oncolytic properties of HSV-1 to directly attack cancer cells, but also stimulates a comprehensive immune response through the immune-enhancing effects of GM-CSF, thereby improving the therapeutic effectiveness of cancer.
Clinical data for the RP1 vaccine came from 140 melanoma patients in IGNYTE's solid tumor tests. The patients' conditions worsened after receiving anti-PD-1 therapy (possibly combined with anti-CTLA-4 drugs) for at least two months. Most patients received first-line (45.7%) or two-line (18.6%) therapy. The expression of PD-L1 in 56.4% of patients was lower than 1%, and 65.7% of patients had initial resistance to PD-1, which may truly reflect the patients with advanced melanoma in the real world.
In June, Replimune revealed that RP1 combined with PD-1 drug Opdivo (nivolumab) of Bristol Myers Squibb (BMS) achieved an overall response rate of 33.6% after one year of use. This suggests that the combination therapy has slightly outperformed an approved innovation in this field, Iovance Biotherapeutics' tumor infiltrating Lymphocyte (TIL) therapy Amtagvi (lifileucel). Amtagvi achieved an overall response rate of 32.9% in Phase II C-144-01 test (according to RECIST 1.1), which prompted the FDA to grant it accelerated approval for the treatment of melanoma that failed PD-1 therapy in February 2023. Therefore, Amtagvi has become an important alternative in the treatment of melanoma after the failure of PD-1 therapy, especially for the advanced patients who are resistant to the existing immune checkpoint inhibitors. At present, RP1 combined with Opdivo gives better data than Amtagvi, which is an important step on the basis of PD-1 inhibitors and can also be regarded as an important breakthrough in PD-1 combination therapy. In terms of safety, RP1 therapy had a sound tolerability, and only 12.8% of patients had grade 3-4 adverse events. Grade 4 adverse events included lipase elevation, cytokine release syndrome, myocarditis, hepatolysis and spleen rupture in one case each. No deaths were reported, again giving RP1 an advantage over Amtagvi, which carries a black box warning of treatment-related death, along with long-term severe cytopenia, severe infection, and cardiopulmonary and renal dysfunction.
Replimune plans to submit the BLA application for RP1 at the end of this year, and strive for the accelerated approval of FDA. At the same time, they have initiated patient enrollment for a confirmatory test of the melanoma trial IGNYTE-3, and on August 13, they injected the first subject to further evaluate the effect of the combination of RP1 and Opdivo in patients with advanced melanoma whose disease has progressed after treatment with PD-1 inhibitors and CTLA-4 inhibitors.
Different from PD-1 combination therapy, PD-1 inhibitors also show a trend of breaking through the cage on the road of bispecific antibody. The bispecific antibody ivonescimab of Summit Therapeutics and Akeso Inc achieved remarkable results in the second phase of clinical trials, and directly challenged the king of medicine, Keytruda.
Ivonescimab, a bispecific antibody, targets PD-1 and VEGF at the same time, which has shown strong efficacy in patients with head and neck cancer, colorectal cancer and breast cancer. On September 16, 2024, Akeso presented the Phase II research data of ivonescimab at the annual meeting of the European Society for Medical Oncology (ESMO) held in Barcelona, Spain, which involved the effect of combining ivonescimab with chemotherapy or CD47 antibody ligufalimab, targeting the first-line treatments of microsatellite-stabilized colorectal cancer (MSS CRC), recurrent/metastatic head and neck squamous cell carcinoma (HNSCC) and triple negative breast cancer (TNBC).
What is more striking about Ivonescimab is that in its Phase III Harmony-2 study, Ivonescimab showed obvious advantages over Keytruda single drugs. The Chinese clinical research showed that ivonescimab reduced the risk of disease progression or death by 49% in PD-L1-positive patients, achieving the first time that Keytruda's competitors have surpassed the first-line treatment for advanced NSCLC. HARMONi-2 study showed that ivonescimab was superior to Keytruda in four main subtypes, including low PD-L1, high PD-L1, squamous and non-squamous patients.
After a median follow-up of nearly nine months, patients treated with ivonescimab had a median progression-free survival of 11.14 months, compared to just 5.82 months in the Keytruda group. Compared to Keytruda, ivonescimab reduced the risk of disease progression or death by nearly 50%, an effect that was statistically significant, with a P-value of less than 0.0001. This finding was also validated in various subgroup analyses, including patients with high and low PD-L1 protein expression levels, as well as squamous and non-squamous disease groups. Ivonescimab also outperformed Keytruda in key secondary endpoints. The overall response rate of patients treated with Ivonescimab was 50%, while that of Keytruda group was 38.5%. DCR in the two treatment groups were 89.9% and 70.5%, respectively.
The challenge of Ivonescimab is that Summit needs to match these data results with global research results. The company plans to conduct a global Phase III study to obtain approvals in the United States, Europe and other key international markets. Given that Keytruda is expected to face a patent cliff in 2028, MSD believes ivonescimab's impact on Keytruda may not be significant.
Ivonescimab has been approved for the first time in China as a second-line treatment for the mutation from EGFR to NSCLC. Its HARMONi-3 study compared ivonescimab in combination with chemotherapy and head-to-head compared with Keytruda plus chemotherapy, but was limited to patients with squamous NSCLC, who make up 25-30% of the total, due to their greater unmet need relative to non-squamous patients.
Following the results, analysts raised the approval probability for ivonescimab and increased its sales forecast, expecting peak sales of USD2.2 billion in HNSCC (head and neck squamous cell carcinoma) and total peak sales of USD15.7 billion across all indications by 2040.
The excellent effect of Ivonescimab compared with Keytruda may have a far-reaching impact on the field of PD-1 immune checkpoint inhibitors. Ivonescimab showed its potential as a bispecific antibody against PD-1 and VEGF, especially in non-small cell lung cancer (NSCLC). This dual mechanism of action may allow ivonescimab to surpass the therapeutic effect of drugs targeting PD-1 alone. This breakthrough suggests that only PD-1 inhibit may not be enough to fully harness the immune system's ability to fight cancer, and that in some cases, suppression combined with other mechanisms may be more effective. This could drive improvements to existing PD-1 inhibitors, or spur the development of new drugs that combine different targets to enhance therapeutic effects.
As the veteran of PD-1 inhibitors, Astrazeneca's Imfinzi (durvalumab) has also recently redeemed itself, getting back on its feet after stumbling on the road to the bladder cancer market.
In 2017, Imfinzi obtained the first accelerated approval from FDA for locally advanced or metastatic urothelial carcinoma with Phase I/II research, but then failed in the Phase III confirmatory test DANUBE in 2020, which led AstraZeneca to withdraw its application for this indication in 2021. Meanwhile, rival checkpoint inhibitors Keytruda and Opdivo have seized the market in the field of bladder cancer, showing benefits of improving disease-free survival in adjuvant treatment for patients at high risk for bladder cancer recurrence.
At the end of June, the resounding Astrazeneca announced top-line data for event-free survival and overall survival from Imfinzi's NIAGARA test. In this study, patients received Imfinzi in combination with chemotherapy before radical cystectomy and Imfinzi as adjuvant treatment after surgery compared with neoadjuvant (i.e., pre-operative) chemotherapy plus surgery in approximately 1,000 patients with muscle-aggressive bladder cancer. At the ESMO 24 congress, AstraZeneca presented data showing that perioperative use of Imfinzi reduced the risk of disease progression, recurrence, omission of surgery, or death by 32% compared to neoadjuvant chemotherapy. For the key secondary endpoint of overall survival, perioperative Imfinzi reduced the risk of death by 25% compared to the combination of neoadjuvant chemotherapy followed by radical cystectomy.
The PD-1 inhibitor that created oncology miracles is far from reaching its limits, with ample room for further innovation. AstraZeneca's PD-1 inhibitor Zynyz (retifanlimab) has achieved a breakthrough in the treatment of a rare cancer - unresectable advanced squamous cell anal cancer (SCAC).
According to the results of the Phase 3 POD1UM-303 test presented by AstraZeneca at ESMO 24, Zynyz combination chemotherapy reduced the risk of progression or death in previously untreated patients with unresectable advanced squamous cell anal canal cancer by 37%, significantly improving patient survival compared to chemotherapy alone. Notably, in October 2021, Incyte had withdrawn its BLA application for Zynyz for second-line treatment after chemotherapy in anal cancer, primarily due to low tumor response rates and a number of patient deaths unrelated to disease progression, and this reboot is a reward for perseverance in this rare field of oncology.
Anal cancer is not a large tumor market from a financial perspective. Zynyz's target patient population is approximatand Europe.ely 10,000 newly diagnosed cases per year in the United States and Europe. Incyte is planning to apply to the FDA for approval of Zynyz before the end of the year, putting it on track to be the first PD-1 inhibitor used in the treatment of first-line anal cancer. This puts Zynyz within a niche indication where it may have some payoff for Incyte.
Zynyz was approved by the FDA into the market last year for Merkel cell carcinoma (another rare type of cancer). Furthermore, Incyte announced positive results for Zynyz in the POD1UM-304 test combined with chemotherapy for first-line non-small cell lung cancer (NSCLC).
PD-1 inhibitors have shown significant efficacy in a variety of tumor types by blocking the PD-1/PD-L1 pathway and restoring the antitumor activity of T cells. However, while PD-1 inhibitors have demonstrated long-term anti-tumor effects in some patients, many patients do not respond to monotherapy or develop resistance after treatment. Accordingly, researchers and pharmaceutical companies are increasingly exploring combination therapies of PD-1 inhibitors with other treatments to broaden their applicability, improve therapeutic effect, and overcome drug resistance.
For instance, Keytruda, the blockbuster PD-1 inhibitor, received its seventh FDA approval for a combination therapy (first-line treatment for metastatic non-squamous NSCLC in 2017) after its initial approval in 2014. Subsequently, Keytruda has garnered 17 FDA approvals for combination therapies, including carboplatin and paclitaxel (June 17, 2024, for primary advanced or recurrent endometrial cancer), chemoradiation (January 12, 2024, for cervical cancer), Padcev (enfortumab vedotin-ejfv, an antibody-drug conjugate, December 15, 2023, for advanced bladder cancer), gemcitabine and cisplatin (November 1, 2023, for locally advanced unresectable or metastatic cholangiocarcinoma), and more.
MSD also presented the latest combination therapy clinical data for Keytruda at ESMO 24. The KEYNOTE-A18 Phase III test enrolled 1,060 women with newly diagnosed high-risk locally advanced cervical cancer who received Keytruda in combination with chemoradiation therapy (CRT). The FDA granted approval for the Keytruda/CRT combination for treating stage III-IVA cervical cancer in women based on the first interim analysis in January this year. The newly released data represent the second interim analysis. The data showed that after a median follow-up of 29.9 months, the 36-month OS rate was 82.6% in the Keytruda/CRT group compared to 74.8% in the CRT mono group. In addition to this, MSD also announced OS data from the KEYNOTE-522 Phase III test of Keytruda in combination with radiotherapy for triple-negative breast cancer (TNBC). After a median follow-up of 75.1 months, the Keytruda regimen reduced patients' risk of mortality by 34%, and the five-year OS rate was 86.6% in the Keytruda group compared to 81.7% in the control group, when compared to placebo plus chemotherapy with postoperative maintenance placebo treatment.
Keytruda's ongoing efforts to pioneer the use of combination therapies represents the future development of PD-1, a trend that stems from the following considerations:
· Enhanced antitumor response: Combination therapies can enhance the immune system's attack on cancer cells through multiple mechanisms. For example, the combination of PD-1 inhibitors with chemotherapy, radiotherapy or other targeted drug can further activate the immune response by stimulating the release of tumor antigens or altering the tumor microenvironment.
· Overcoming immune evasion: when PD-1 inhibitors are used alone, certain tumors evade recognition by the immune system or suppress immune system activity through multiple pathways. Combining other immune checkpoint inhibitors (e.g., CTLA-4 inhibitors) or other types of immunotherapies (e.g., lysoviruses, CAR-T cell therapy, etc.) can block additional mechanisms of tumor escape, thereby improving the immune system's ability to fight tumors.
· Expanding the range of indications: PD-1 inhibitors exhibit varying sensitivities across different tumor types. Combining PD-1 inhibitors with other therapies can expand their applicability. For instance, certain tumors may not respond to PD-1 inhibitor monotherapy but may become sensitive when combined with chemotherapy, radiation therapy, or angiogenesis inhibitors.
· Reducing drug resistance: man patients develop resistance after receiving PD-1 inhibitor treatment. Combining PD-1 inhibitors with other treatment modalities can delay or prevent the development of resistance. For example, combining PD-1 inhibitors with anti-angiogenic drugs can modify the tumor microenvironment, making it more permissive for T-cell infiltration and killing, thus overcoming resistance.
· Improved efficacy and survival: a growing number of clinical trials have demonstrated that combination therapies are more effective than monotherapy in multiple cancer types treatment. For example, the combination of a PD-1 inhibitor with a CTLA-4 inhibitor has shown significant prolongation of survival and improved disease control in tumors such as melanoma and NSCLC.
The combination of PD-1 inhibitor with other therapies opens up new horizons in antitumor therapy, providing more therapeutic options and promising to overcome the limitations of monotherapy and benefit more patients. This combination strategy will have an important role in future tumor therapy.
PD-1 inhibitors, represented by Keytruda and Opdivo, have achieved remarkable results in the field of tumor immunotherapy, successfully changing the treatment landscape for a variety of cancers. However, the development potential of PD-1 therapies remains enormous. First, the indications for PD-1 inhibitors are expected to be further expanded, and patients with more cancer types and earlier stage tumors may benefit from them. Second, the combination of PD-1 inhibitors with other novel therapies, such as with cell therapy, tumor vaccines, or other immune checkpoint inhibitors, has the potential to enhance efficacy and overcome drug resistance. In addition, the continuous optimization of biomarkers will help screen beneficiary patients more accurately and enhance the individualized therapeutic effect. As technology and research advance, the role of PD-1 inhibitors in treating complex tumors will become more widespread, with ample room for future growth and development.
Sava, J. RP1 Plus Nivolumab Demonstrates Durable Antitumor Activity in Advanced Melanoma. OncLive. 15. 09. 2024.
Cairns, E. ESMO 24: Replimune’s Cancer Vaccine Succeeds In PD-1 Failures. Scrip. 16. 09. 2024.
Manalac, T. Summit Declares NSCLC Victory Over Keytruda, Analysts Advise Caution With China-Only Data. Biospace. 09. 09. 2024.
Liu, A. ESMO: Even after unique trial win, Incyte CEO views PD-1 drug Zynyz as pipeline aide. Fierce Pharma. 14. 09. 2024.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


