Neeta RatanghayraOctober 16, 2024
Tag: Biology , Challenges
Synthetic biology includes the design, construction, and redesign of biologically based components, systems, and natural biological systems for various applications. Over recent decades, significant progress has been achieved in crafting delicate biocircuits, standardizing biological building blocks, and developing various genomic/metabolic engineering tools and approaches.
Recognized for its transformative potential, synthetic biology has emerged as a technology poised to revolutionize various industries, including pharmaceuticals. Areas, where synthetic biology can be used, include integrating heterologous pathways into designer cells to streamline the production of medical agents, enhancing yields of natural products in cell growth media to equal or exceed those obtained from traditional sources, and pioneering novel genetic circuits for targeted tumor therapies. Synthetic biology is also driving innovations in controlled drug release systems, tailored to respond to specific biomarkers, thereby enhancing treatment efficacy for diseases such as diabetes and cancers. Additionally, new strategies are being devised to tackle complex immune disorders, infectious diseases, and metabolic disorders that pose challenges for conventional treatment approaches.
Synthetic biology involves dismantling and reassembling biological cells and processes to create innovative systems. This process begins with the encoding of designs by deoxyribonucleic acid (DNA), which serves as the blueprint for biological parts. These biological parts, or bioparts, are then combined to form devices, which are subsequently integrated into biological systems.
Though a diverse field, synthetic biology can broadly be divided into two main approaches: bottom-up and top-down methodologies. Bottom-up approaches aim to create artificial life from scratch, while top-down approaches leverage known biology to design systems for specific tasks. The latter approach involves designing metabolic and signaling pathways within cells to achieve desired objectives.
The advancements in DNA sequencing and synthesis technologies, coupled with insights from systems biology, have fueled the growth of synthetic biology. However, the emergence of RNA therapeutics has sparked interest in harnessing the unique attributes of RNA molecules. RNA-based systems, constructed using diverse libraries, offer enhanced safety profiles compared to DNA-based systems, making them suitable for therapeutic applications with stringent safety standards. Additionally, RNA-based systems exhibit rapid action, as they do not require transcription.
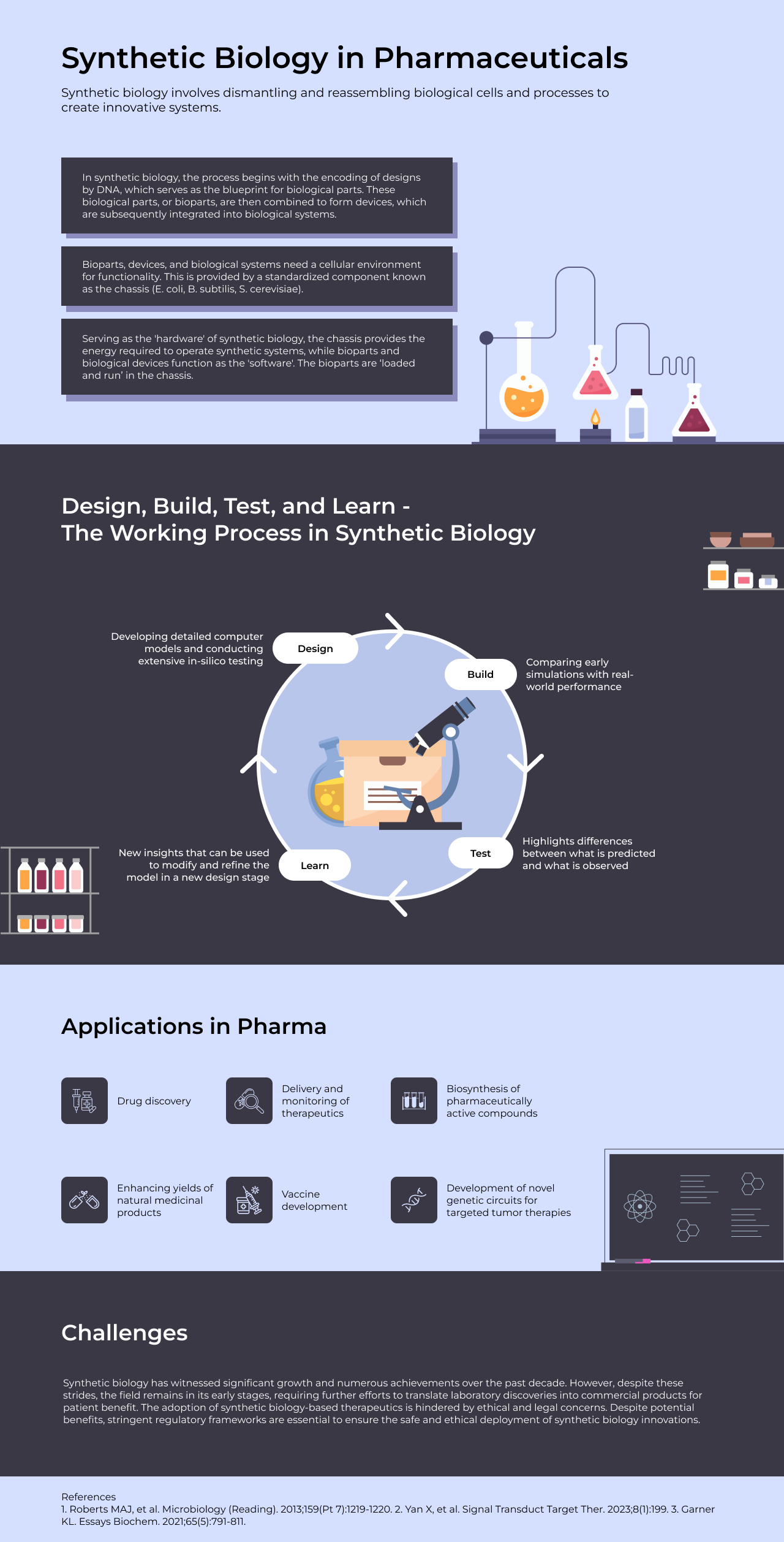
Working process in synthetic biology follows an iterative cycle of Design, Build, Test, and Learn. Design stage, the first stage, involves developing detailed computer models and conducting extensive in silico testing. Comparing early simulations with real-world performance in the Build stage highlights differences, which are then explored in the Test stage to gain new insights. These insights can be used to modify and refine the subsequent Design stage, perpetuating a cycle of iterative improvement until the desired biological outcome is achieved.
Critical to the construction of biological devices and systems is the compatibility and ease of assembly of bioparts. Ensuring that bioparts can seamlessly integrate as modules with minimal optimization is imperative for successful implementation.
Bioparts, devices, and biological systems need a cellular environment for functionality. In synthetic biology, the cellular environment can be furnished by a standardized component known as the chassis. Serving as the 'hardware' of synthetic biology, the chassis provides the energy required to operate synthetic systems, while bioparts and biological devices function as the 'software'. Escherichia coli (E. coli) has historically served as the model organism due to its well-established characteristics and is hence the go-to chassis for synthetic biology. However, alternative chassis organisms such as Bacillus subtilis (B. subtilis) and yeast, such as S. cerevisiae, are also utilized based on specific requirements. The choice of chassis profoundly influences the performance of bioparts, hence it should be well-characterized and have minimal unwanted interactions to ensure optimal system efficiency. Ideally, a minimal cell chassis harboring the fewest genes necessary to support metabolism is preferred.
While a chassis provides the hardware for synthetic systems, it can be difficult to control leading to several unforeseen side reactions. In some cases, removing the cellular environment entirely to create a cell-free system can be advantageous, particularly for pharmaceutical manufacturing. Cell-free systems offer scalability and flexibility, facilitating the development of viable industrial processes with reduced risk of unwanted side reactions.
Synthetic regulatory circuits are bioparts designed to perform (just like electronic circuits) logical functions within biological systems. These circuits can detect various biomolecules through appropriate sensors and can be tuned to identify specific pathogens or disease markers. While traditional sensory circuits operate within a cellular chassis, advancements in synthetic biology have enabled the development of cell-free systems for enhanced versatility and adaptability.
To facilitate efficient data exchange within the synthetic biology community, a standardized data format known as Synthetic Biology Open Language (SBOL) has been developed. SBOL enables the representation of biological designs in silico, streamlining collaboration and knowledge sharing among scientists.
Synthetic biology has emerged as an efficient tool for the production of cost-effective pharmaceutical agents. One notable success story in this realm is the production of artemisinin, a vital treatment for malaria. While artemisinin is naturally derived from sweet wormwood, its precursor, artemisinic acid, can be synthesized in Saccharomyces cerevisiae (baker’s yeast) through metabolic engineering. By introducing the necessary genes into yeast cells, the pharmaceutical company Sanofi has significantly improved the yield of artemisinin, marking a milestone in the field of metabolic engineering.
Beyond enhancing drug production, synthetic biology has revolutionized therapeutic approaches for hard-to-treat cancers, as evidenced by the approval of Kymriah by Novartis. Kymriah represents the first therapy utilizing engineered living cells to receive approval from the U.S. Food and Drug Administration. This groundbreaking treatment targets B-cell acute lymphoblastic leukemia (ALL), a prevalent childhood cancer affecting the immune system's B lymphocytes. Through genetic modification, patients' T cells are engineered to express chimeric antigen receptors (CARs) that specifically recognize malignant B cells, enabling targeted destruction of cancerous cells by the immune system. CAR-T cell therapy has demonstrated remarkable efficacy offering a promising agent for treating ALL.
Synlogic, a pharmaceutical company, has leveraged metabolic engineering to enable Escherichia coli Nissle 1917 to metabolize phenylalanine, offering a potential treatment for phenylketonuria (PKU). By breaking down phenylalanine into non-toxic molecules, this engineered bacterium holds significant promise for alleviating the symptoms of PKU. This treatment, Labafenogene marselecobac, is currently in phase 3 clinical trials.
The recent success of mRNA COVID-19 vaccines has propelled interest in RNA therapeutic technology, highlighting the transformative potential of synthetic biology in addressing global health challenges. RNA vaccines, designed to introduce synthetic RNA-encoding pathogen-specific antigens into cells, offer advantages in terms of speed, scalability, safety, and immunogenicity over conventional vaccines. The synthetic production method enables rapid large-scale manufacturing, making mRNA vaccines particularly suited for combating emerging infectious diseases like COVID-19.
Synthetic biology has witnessed significant growth and numerous achievements over the past decade. However, despite these strides, the field remains in its early stages, requiring further efforts to translate laboratory discoveries into commercial products for patient benefit.
While synthetic biology primarily focuses on microbial systems, addressing human health challenges necessitates advancements in mammalian synthetic biology. This includes the development of next-generation treatments, such as synthetic gene networks for disease management, tissue engineering, and stem cell generation.
Furthermore, the adoption of synthetic biology-based therapeutics is hindered by ethical and legal concerns. Despite potential benefits, stringent regulatory frameworks are essential to ensure the safe and ethical deployment of synthetic biology innovations.
1. Roberts MAJ, Cranenburgh RM, Stevens MP, Oyston PCF. Synthetic biology: biology by design. Microbiology (Reading). 2013;159(Pt 7):1219-1220.
2. Yan X, Liu X, Zhao C, Chen GQ. Applications of synthetic biology in medical and pharmaceutical fields. Signal Transduct Target Ther. 2023;8(1):199.
Garner KL. Principles of synthetic biology. Essays Biochem. 2021;65(5):791-811.
Freelance Medical Writer
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


