Saher HaiderOctober 15, 2024
Tag: Cryogenic Cold Chain , Cell Therapy , Logistics
Unlike conventional temperature-sensitive pharmaceutical products, cell therapy products have the most stringent requirements for cold chain logistics. In this article , we gave you a brief overview of what cell therapy products are, the logistical challenges these products pose, and best practices for maintaining cell therapy cold chain.
In this article, we will dive deeper into cryogenic technology used at every step of the cold chain supply chain.
So, without further ado, let’s dive right into the article!
Cryogenic Cold Chain Technology refers to the process of preserving and transporting highly sensitive biological materials, such as cell therapy products, at ultra-low temperatures. The temperatures used in this technology fall below -150°C and can be achieved using liquid nitrogen vapor (-196°C).
What makes cryogenic cold chain technology unique, and challenging is that, unlike standard cold chain systems that use refrigeration temperatures (2-8°C) or freezing temperature (-20°C), cryogenic cold chains are designed to maintain cellular integrity and functionality over extended periods by halting biological activity and putting the cells into a suspended state.
Cell therapy products are derived from live mammalian cells, which makes them particularly sensitive to temperature fluctuations. At cryogenic temperatures, cellular metabolic processes are nearly completely stopped, preventing cellular degradation and death during storage and transportation. Thus, cryogenic cold chain technology extends the shelf life of these perishable products, enabling long-term storage and transportation without losing their viability.
Cryogenic conditions also allow for the decoupling of manufacturing from clinical administration, reducing the time pressures of "just-in-time" logistics, which currently dominate the supply chain for cell and gene therapies.
While cryogenic cold chain technology is still developing, traditional cold chain methods are already well-established for biologics like vaccines, monoclonal antibodies, and other temperature-sensitive pharmaceuticals. However, knowing the key differences between the two is important for understanding the need for developing cryogenic methods for cell therapy products.
In the following table, we have summarized how both these methods compare in different aspects, including temperature range, storage duration, handling complexity, application, cost and infrastructure.
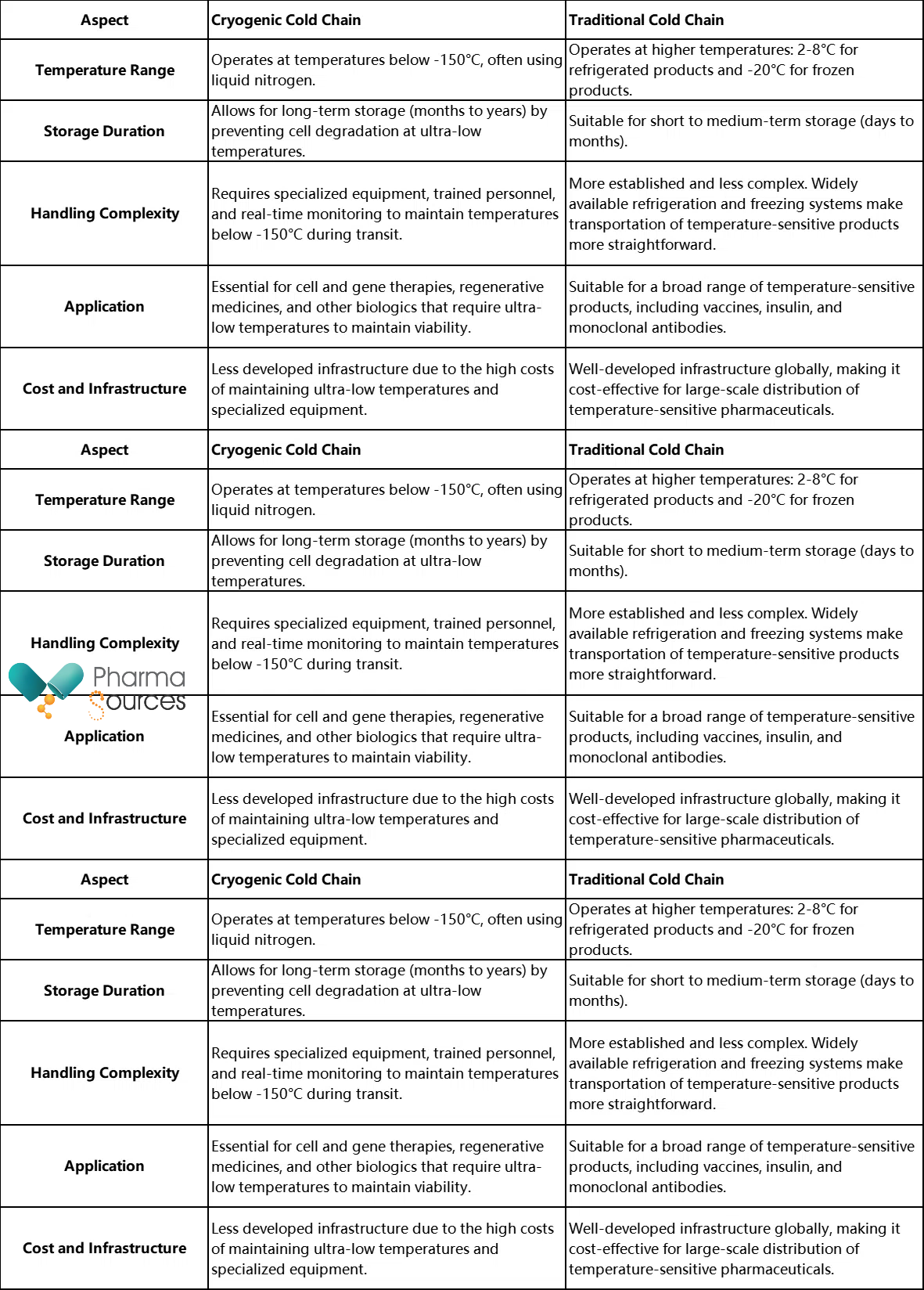
We know for a fact that, unlike traditional cold chains, cryogenic systems are specifically developed to maintain ultra-low temperatures (below -150°C). However, the development of these systems faces significant technological and commercial challenges.
Some of the most common elements used for the development of cryogenic cold chain technology throughout the supply chain are:
Liquid Nitrogen (LN2) storage freezers are the most common type of cryogenic freezers used in cell therapy storage. LN2 storage freezers are cylindrical stainless-steel vessels, the bottom of which are filled with liquid nitrogen. The resulting vapor phase nitrogen fills the rest of the chamber, where the product is placed above the liquid level in racks. Temperatures inside the chamber vary, with the lowest temperatures of around -190°C at the bottom to the highest, around -120°C at the top. This temperature variation in LN2 storage freezers is critical as products placed higher in the chamber are warmer than those stored lower.
The biggest advantage of LN2 freezers is their ability to maintain ultra-low temperatures without electrical power for extended periods—often for days, making them ideal for situations where power outages or supply chain disruptions may occur.
However, LN2 freezers also come with challenges, such as handling complexity and the need for specialized infrastructure to ensure safe storage without direct contact between LN2 and the stored product.
When the LN2 supply is unavailable, Ultra-Cold Mechanical Freezers may be used to maintain cryogenic temperatures. These are chest-style freezers that store products in the bottom section of the chamber to maintain temperature uniformity and reduce temperature excursions when the freezer door is opened. The only and most obvious downside of these freezers is that they depend on a constant power supply. Thus, in the event of power failure, they warm up much faster than LN2 storage freezers, posing a risk to stored products.
Dry shippers are often used for cryogenic shipping during air transit. These shippers consist of an absorptive cooling medium that is charged with LN2 before excess LN2 is removed. The medium then sublimates vapor phase nitrogen to cool the internal chamber, allowing safe transport of biologics without the risk of direct contact with LN2. Dry shippers are available in various sizes, ranging from small units capable of holding up to 1,000 vials to large-scale palletized systems accommodating up to 20,000 vials.
Although dry shippers are compliant with air travel regulations, they have limitations. The orientation of the shipper during transport is critical—if not kept upright, vapor loss accelerates, which can reduce the hold time at cryogenic temperatures.
For large-scale shipping where standard dry shippers are insufficient, cryogenic storage freezers can be modified for transport. These palletized freezers protect the stored product from direct contact with LN2 and prevent spillage during transit. Palletized storage freezers are ideal for long-distance transport of large quantities of cell therapy products but require careful handling to ensure that the LN2 remains secure and that the temperature inside the chamber stays consistent.
Mobile storage freezers are used for the transport of large quantities of cryogenically stored products. They are equipped with casters for mobility, but movement introduces the risk of LN2 sloshing and contacting the product. To address this, isothermal designs are used to separate the LN2 from the internal storage chamber, reducing the risk of contamination and allowing safer transport.
Sample carriers serve as a portable solution for the local transport of cryogenically frozen products within hospitals or research facilities. These are compact, insulated carriers that come with temperature monitoring systems and audible alarms to alert users if the internal temperature rises above acceptable limits. Sample carriers are best suited for transporting small quantities of vials or samples over short distances.
Portable cryo-workbenches provide a controlled environment to prevent the products from warming above critical thresholds during the handling of cryogenically frozen cell therapy products. These workbenches include a raised platform within an insulated chamber, cooled by a refillable reservoir of LN2. The design allows users to safely package products for shipment, conduct secondary processing, or perform investigations without risking temperature excursions.
Cryogenic cold chain technology has seen remarkable advances in recent years due to the growing demand for transporting and storing sensitive biological materials like cell therapies. Companies such as Cryoport and Stirling Ultracold are already offering high-performance containers that provide consistent temperatures as low as -150°C while ensuring product safety and compliance during transit. These containers minimize the risks associated with cryogenic shipments by maintaining ultra-low temperatures for extended periods, even under challenging conditions.
In parallel, real-time temperature monitoring systems enable precise control during the transport of temperature-sensitive products. Advanced sensors and data loggers, like those from Berlinger and Sensitech, are designed for ultra-low temperature environments that detect temperature excursions instantly to maintain product integrity.
Another key advancement is automation, with robotics playing a prominent role in the storage, handling, and retrieval of cell therapy products. Companies like Brooks Life Sciences have developed automated cryogenic storage facilities that enhance efficiency, minimize human error, and maintain consistent ultra-low temperatures.
Finally, sustainability efforts are gaining traction, with innovations such as energy-efficient freezers and recyclable cryogenic materials. For instance, Cryoport’s reusable containers reduce waste and energy consumption while maintaining high performance, aligning with global sustainability goals.
The development of cryogenic cold chain technology is essential for the preservation and distribution of cell therapy products, which require ultra-low temperatures to maintain cellular viability. Technological innovations like LN2 storage freezers, ultra-cold mechanical freezers, and dry shippers have improved storage and transport capabilities, while automated systems reduce human error. Real-time temperature monitoring and sustainability-focused designs further improve the reliability and environmental impact of cryogenic logistics. As cell and gene therapies become more prevalent, these advancements will influence the future of pharmaceutical logistics, allowing for the safe delivery of life-saving treatments.
Saher Binte Haider is a pharmacy graduate from Dow University of Health Sciences. She started her career as a Quality Management professional in the pharmaceutical industry where she developed a keen interest in good documentation practices, SOP creation, and content writing. She has 7+ years of experience in healthcare & life sciences content writing. Her key areas of expertise are healthcare, pharmaceuticals, health tech, and AI in healthcare.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


