PharmaSources/ChatGPTAugust 28, 2024
Tag: Semaglutide , Weight-Loss Market , Chinese Innovations
In recent months, Semaglutide has surged into the global spotlight as a groundbreaking solution for weight loss, captivating attention from both consumers and industry experts alike. Notably, even high-profile figures like Elon Musk have publicly shared their success stories with Semaglutide, highlighting its effectiveness in managing weight. This article is tailored for professionals in the pharmaceutical industry interested in exploring international market opportunities, including roles such as business development managers, regulatory affairs specialists, and market access strategists. Understanding the implications of Semaglutide’s rise not only offers valuable insights into current market trends but also equips industry stakeholders with the knowledge necessary to navigate the evolving landscape of obesity treatments and the global weight-loss market.
Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist used to manage type 2 diabetes and aid in long-term weight management. It is available as both a subcutaneous injection and an oral tablet, marketed under the names Ozempic and Rybelsus for diabetes, and Wegovy for weight loss.
Semaglutide Chemical Structure
Provided by AI(ChatGPT)
Type 2 Diabetes: Improves glycemic control in conjunction with diet and exercise.
Obesity: Used for weight management in adults with a BMI ≥ 30 or ≥ 27 with weight-related health conditions.
Expanded FDA approval in 2024 includes reducing cardiovascular risks.
Increased risk of nutritional deficiencies and metabolic disorders.
Potential digestive system issues.
Possible neurological and psychological effects.
Nausea
Vomiting
Diarrhea
Constipation
Abdominal pain
Headache
Fatigue
GERD
Hypoglycemia (for diabetes patients)
Pancreatitis
Gastroparesis
Semaglutide was developed by Novo Nordisk, following earlier research on GLP-1 hormones. Novo Nordisk’s work led to the creation of liraglutide before semaglutide, further advancing diabetes and obesity treatments.
In a March 2021 phase III trial involving 1,961 adults with high BMI, semaglutide resulted in a 14.9% average weight loss compared to 2.4% with a placebo. Semaglutide showed more promise than previous anti-obesity drugs but was less effective than bariatric surgery.
US: Ozempic costs $936 per month; Wegovy costs $1,349.02 per month.
UK: Available on NHS for diabetes at minimal or no cost, and for obesity limited to two years of treatment.
Europe: Prices range from $87 in Australia to $144 in Switzerland. France has the lowest at $83.
According to a recent report by Fortune Business Insights, the global weight loss drug market is projected to grow significantly from $4.51 billion in 2023 to $22.85 billion by 2030, with a compound annual growth rate (CAGR) of 26.1%. Additionally, global weight loss medications are expected to reach approximately $100 billion by 2030.
The demand for weight loss solutions is surging worldwide. For instance, semaglutide-based products, including Ozempic (for diabetes), Rybelsus (oral diabetes medication), and Wegovy (for weight loss), have seen impressive sales. Wegovy, in particular, achieved sales of $1.759 billion in the first half of 2024, reflecting a dramatic 367% increase from the previous year.
The FDA has approved several drugs for long-term weight management, including orlistat (Xenical, Alli), phentermine-topiramate (Qsymia), naltrexone-bupropion (Contrave), liraglutide (Saxenda), semaglutide (Wegovy), and tirzepatide (Zepbound). Four of these treatments are authorized for use in adults and children aged 12 and older, marking significant progress in obesity management.
In China, the market for GLP-1 (glucagon-like peptide-1) drugs for obesity is still in its early stages. However, numerous Chinese pharmaceutical companies are actively developing GLP-1-based treatments. The following table summarizes the development stages of various weight loss drugs from Chinese companies:
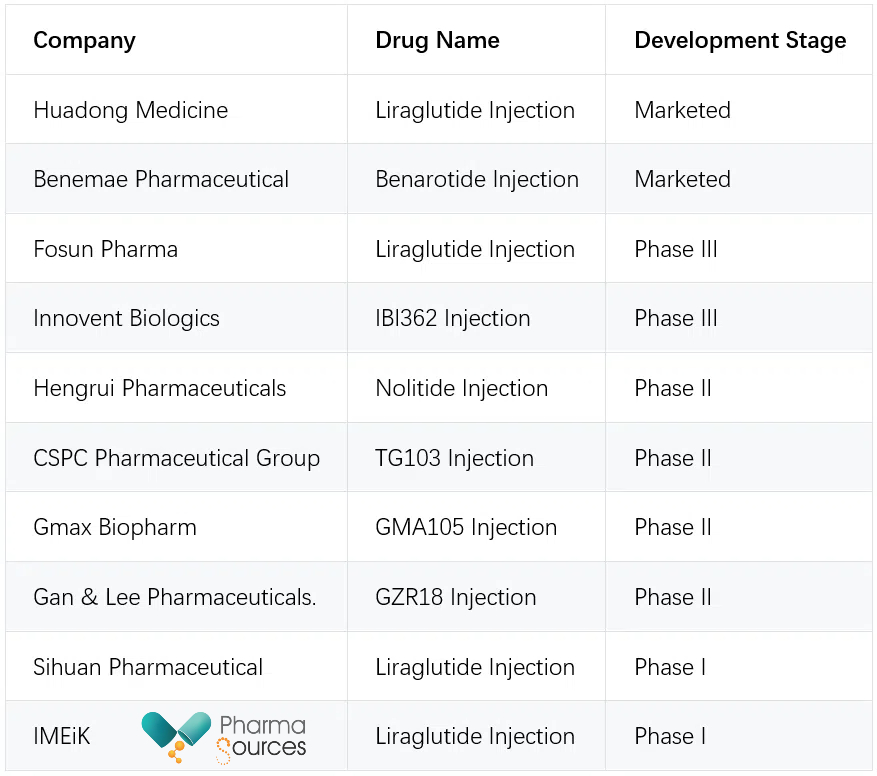
Weight Loss Drugs Development in China
Hengrui has recently licensed out its GLP-1 product portfolio to Hercules, a U.S. company, marking a major step in globalizing its innovative drugs. This deal, valued at up to $6 billion, includes an upfront payment and milestone payments totaling $1.1 billion. Hengrui will also gain a 19.9% equity stake in Hercules. The licensed products include HRS-7535, an oral GLP-1 receptor agonist, HRS9531, a dual agonist of gastric inhibitory polypeptide receptor and GLP-1 receptor, and HRS-4729, a next-generation incretin-based drug.
China has seen a record number of its innovative drugs gain approval in international markets. In 2023 alone, four Chinese-developed drugs received FDA approval, setting a new benchmark for the industry. This trend highlights China’s growing influence in the global pharmaceutical landscape and its increasing role in advancing global health solutions.
1、 Semaglutide - Wikipedia: https://en.wikipedia.org/wiki/Semaglutide
2、 Best practices for prompt engineering with the OpenAI API | OpenAI Help Center:https://help.openai.com/en/articles/6654000-best-practices-for-prompt-engineering-with-the-openai-api
3、 长期注射司美格鲁肽会对身体产生什么影响?https://mp.weixin.qq.com/s/-0H9gKRdn-6EpQhPNaGdoQ
4、 靶点 | 星星之火可以燎原,一文了解减重赛道新靶点研发进展 https://mp.weixin.qq.com/s/gYMb7q5iU7oNce4pt25n-Q
5、 恒瑞60亿美元出海,减肥药领域还有哪些新机会?https://mp.weixin.qq.com/s/UwgTiQRa5SHzeH3tXqTjTg
6、 重磅!减肥药替尔泊肽在中国获批上市,疗效卓越:https://mp.weixin.qq.com/s/iS7yEC9EJoe8ePGytpTAGQ
7、 明星药物减重范围再下调,GLP-1减肥药市场激战正酣 https://mp.weixin.qq.com/s/b7bzeEkmRh9K1E-xLYe37Q
8、 Best practices for prompt engineering with the OpenAI API | OpenAI Help Center https://help.openai.com/en/articles/6654000-best-practices-for-prompt-engineering-with-the-openai-api
9、www.google.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


