Suzanne ElvidgeAugust 02, 2024
Tag: Immunoconjugates , Diagnosis , Treatment
Immunoconjugates – complex molecules that combine a targeting component, a linker molecule and a therapeutic or diagnostic component (also known as a payload) – have been described as magic bullets as they allow the precise delivery of an active molecule to its target. [1]
In the early 1900s, Paul Erlich of the Institute of Experimental Therapy (Institut für experimentelle Therapie) was concerned about the off-target effects seen with the systemic treatment of bacterial infections. He came up with the idea of a ‘magic bullet’ (Zauberkugel); a molecule that could target specific microbes without harming the body itself. The first drug to fit the description of a magic bullet was Erlich’s arsphenamine (Salvarsan), an antimicrobial for treating syphilis that specifically killed the spirochete Treponema pallidum. Salvarsan was launched in 1910 and used until the 1940s when it was supplanted by penicillin. [2, 3]
Throughout the 20th century, the idea that there could really be a magic bullet – a systemically delivered treatment that targeted disease without harming healthy tissue – became a goal for drug developers. This took a major step forward with the 1986 launch of the first monoclonal antibody drug, Orthoclone OKT3 (muromonab-CD3), for the prevention of kidney transplant rejection. [4]
Almost a hundred years after Erlich first used the term, Pfizer/Wyeth's gemtuzumab ozogamicin (Mylotarg) was approved by the FDA (Food & Drug Administration) for the treatment of acute myeloid leukaemia (AML). [5] Using a monoclonal antibody to deliver a drug payload, the immunoconjugate Mylotarg took the concept to a new level – a magic bullet that is closer to a biological missile. [6]
Mylotarg only made sales of $8.8 million in its first quarter, but the market is now huge. In 2023, the global antibody drug conjugates market was worth around $9.7 billion, with potential to grow to $19.8 billion by 2028, growing at a CAGR (compound annual growth rate) of 15.2% from 2023 to 2028. [7, 8]
The ideal target for an immunoconjugate should not be secreted into the circulation, should be highly expressed at the site where the drug is to act, and be expressed only at low levels or absent in other tissues. The targeting molecule, usually an antibody or antibody fragment, is responsible for directing the immunoconjugate to its site of action. The ideal targeting molecule should have high levels of affinity and selectivity for the target, with little or no off-site binding. It should also have a long plasma half-life and have low propensity for immunogenicity. [6, 9]
The choice of active molecules used in immunoconjugates will depend on the indication, and include: [6, 9]
● Drugs, including cytotoxics, cytokines and oligonucleotides
● Radioisotopes (for imaging and therapy)
● Imaging labels
The linker molecule connects the active ingredient and the targeting molecule. It needs to be sufficiently stable in plasma to ensure that the active ingredient reaches its target, and then release it at the right location and time through enzymatic cleavage or chemical degradation. Linkers can be cleavable or non-cleavable, and the choice depends on the environment where the drug is to be released. Examples of cleavable linkers include acid-sensitive, reductive and enzyme-cleavable linkers. Non-cleavable linkers are used when the targeting molecule is broken up by lysosomes after being internalised into the target cell. The linker should not induce immunoconjugate aggregation. [6, 9]
Immunoconjugates can be associated with a range of side effects. Most of these relate to the side effects associated with the payload, for example the expected adverse effects seen with cancer chemotherapeutics. If the antibody engages with targets on healthy cells, payload accumulation at these sites can cause adverse effects. There can also be problems if the linker releases the payload too soon, away from the target. These effects can be mitigated by adjusting the immunoconjugate chemistry, detecting issues sooner, administering prophylactics and treating symptoms. [10]
The most common use of immunoconjugates is in the treatment of cancer. Also known as antibody-drug conjugates (ADCs), the payloads for these are highly potent cytotoxics. [6]
As of October 2023, the FDA had approved 15 antibody drug conjugates in cancer, with three predicted to be launched in 2024 or 2025: [11, 12]
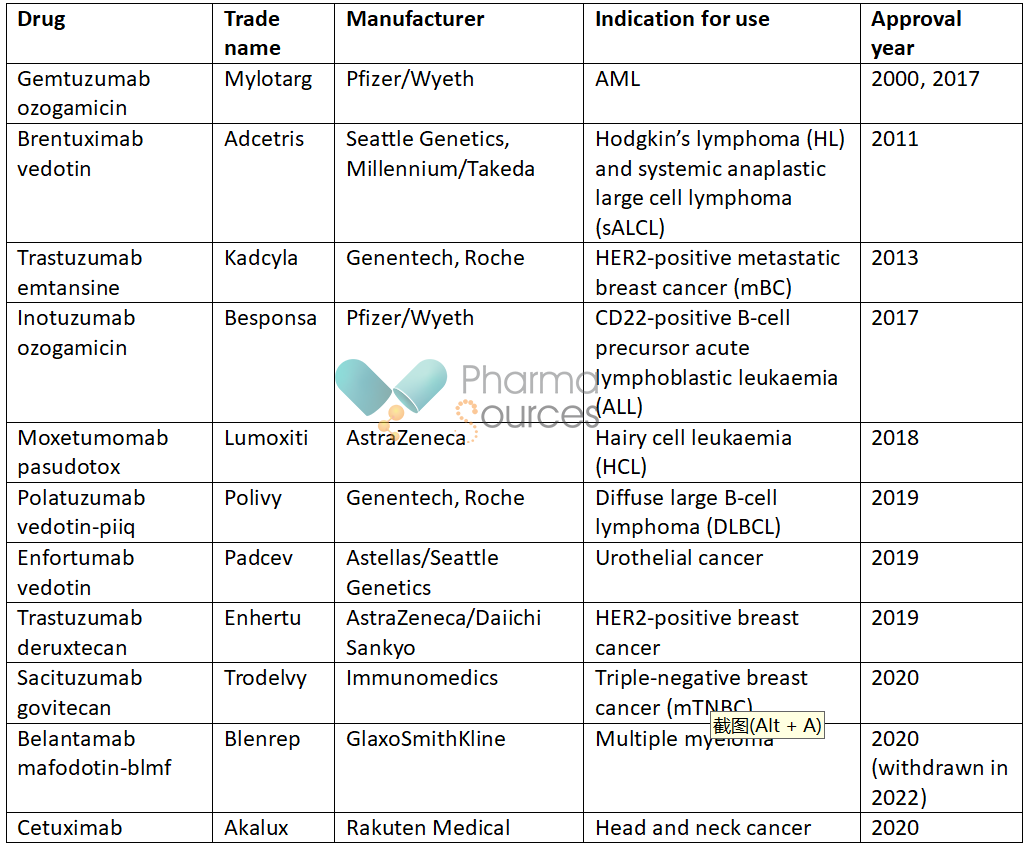
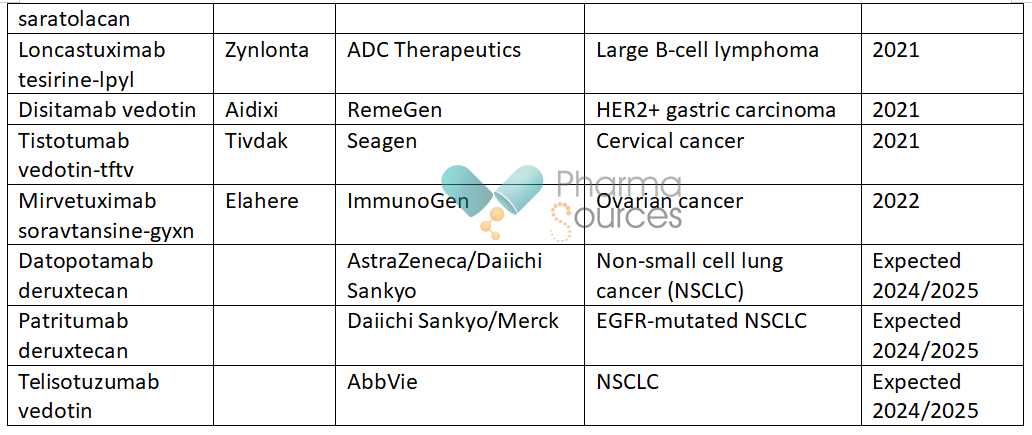
In cancer, immunoconjugates could also incorporate immune-stimulating agents or be given in combination with immunotherapies and other therapeutics. [13] Radiolabelled antibodies (antibody radionuclide conjugates; ARCs) can be used to deliver therapeutic or diagnostic radiation. [14]
A number of companies and academic research teams are looking to use the benefits of immunoconjugates beyond cancer. Potential applications include:
● Antibody-antibiotic conjugates (AACs) to treat bacterial infections, using the specificity of an antibody to a target bacterium to deliver an antibiotic to the site of infection, or to a biofilm on a medical implant such as a catheter [9, 15, 16]
● ADCs to treat autoimmune diseases and immune-mediated inflammatory diseases, using antibodies to deliver glucocorticoids or glucocorticoid receptor modulators to immune cells and sites in inflammation [9, 16-18]
● Antibody-siRNA (small interfering RNA) conjugates, using antibodies to deliver siRNA into cells for autoimmune diseases such as myasthenia gravis and for muscular disorders [16]
Other conditions where ADCs may play a role include glomerular nephritis, rheumatoid arthritis and other rheumatic diseases, atherosclerosis and systemic sclerosis. [16, 19]
In summary, immunoconjugates already play an important role in the treatment of cancer, and their role is likely to expand into other therapeutic indications. Paul Erlich would perhaps have been pleased to see how his concept has been realised.
1. Staff writer. Immunoconjugates. DrugBank. 30 July 2024. Available from: https://go.drugbank.com/categories/DBCAT000152.
2. Erlich, P., Experimental Researches on Specific Therapy: On Immunity with special Reference to the Relationship between Distribution and Action of Antigens. Journal of the Royal Institute of Public Health, 1907. 15(6): p. 321-340.
3. Tan, S. and S. Grimes, Paul Ehrlich (1854-1915): man with the magic bullet. Singapore Medical Journal, 2010. 51(11): p. 842-843.
4. Liu, J.K.H., The history of monoclonal antibody development – Progress, remaining challenges and future innovations Ann Med Surg (Lond), 2014. 3(4): p. 113-116.
5. Staff writer, Pfizer Receives FDA Approval for MYLOTARG™ (gemtuzumab ozogamicin). 1 September 2017. Available from: https://www.pfizer.com/news/press-release/press-release-detail/pfizer_receives_fda_approval_for_mylotarg_gemtuzumab_ozogamicin.
6. Fu, Z., et al., Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther, 2022. 7(1): p. 93.
7. Staff writer, Antibody Drug Conjugates (ADC) Market by Product (Kadcyla, Enhertu, Padcev, Polivy), Linker Type (Cleavable, Non-Cleavable), Payload Type (Calicheamicin, MMAE), Target (HER2, CD30, CD22), Disease, Region - Global Forecast to 2028. Markets and Markets. October 2023. Available from: https://www.marketsandmarkets.com/Market-Reports/antibody-drug-conjugates-market-122857391.html.
8. Staff writer, Pfizer pulls leukemia drug from U.S. market. Reuters, 21 July 2010. Available from: https://www.reuters.com/article/business/healthcare-pharmaceuticals/pfizer-pulls-leukemia-drug-from-us-market-idUSTRE65K5QG/.
9. Sasso, J.M., et al., The Evolving Landscape of Antibody-Drug Conjugates: In Depth Analysis of Recent Research Progress. Bioconjug Chem, 2023. 34(11): p. 1951-2000.
10. Osterweil, N., ADC Toxicities: The Price You Pay for Efficacy. Medscape, 29 September 2023. Available from: https://www.medscape.com/viewarticle/996958.
11. Rosen, D.L., Cancer Drugs (Antibody Drug Conjugates): An FDA Perspective - ADC Blog Series: Article 2. Health Care Law Today, 4 April 2024. Available from: https://www.foley.com/insights/publications/2024/04/cancer-drugs-antibody-drug-conjugates-an-fda-perspective/.
12. Staff writer, Three ADCs Expected To Be Approved In 2024-2025. Biopharma PEG blog, 10 May 2024. Available from: https://www.biochempeg.com/article/397.html.
13. Shastry, M., et al., Rise of Antibody-Drug Conjugates: The Present and Future. Am Soc Clin Oncol Educ Book, 2023. 43: p. e390094.
14. Wei, Z., et al., Engineered Antibodies as Cancer Radiotheranostics. Adv Sci (Weinh), 2024: p. e2402361.
15. Darbandi, A., et al., Antibody-Antibiotic Conjugates: A Comprehensive Review on Their Therapeutic Potentials Against BacterialInfections. J Clin Lab Anal, 2024. 38(10): p. e25071.
16. Pal, L.B., et al., An Overview of the Development and Preclinical Evaluation of Antibody-Drug Conjugates for Non-Oncological Applications. Pharmaceutics, 2023. 15(7).
17. Li, X., et al., An immunomodulatory antibody-drug conjugate targeting BDCA2 strongly suppresses plasmacytoid dendritic cell function and glucocorticoid responsive genes. Rheumatology (Oxford), 2024. 63(1): p. 242-250.
18. Zhang, L., et al., A CD6-targeted antibody-drug conjugate as a potential therapy for T cell-mediated disorders. JCI Insight, 2023. 8(23).
19. Huang, Z., et al., Precision Medicine in Rheumatic Diseases: Unlocking the Potential of Antibody-Drug Conjugates. Pharmacol Rev, 2024. 76(4): p. 579-598.
Based in the north of England, Suzanne Elvidge is a freelance medical writer with a 30-year experience in journalism, feature writing, publishing, communications and PR. She has written features and news for a range of publications, including BioPharma Dive, Pharmaceutical Journal, Nature Biotechnology, Nature BioPharma Dealmakers, Nature InsideView and other Nature publications, to name just a few. She has also written in-depth reports and ebooks on a range of industry and disease topics for FirstWord, PharmaSources, and FierceMarkets. Suzanne became a freelancer in 2006, and she writes about pharmaceuticals, consumer healthcare and medicine, and the healthcare, pharmaceutical and biotechnology industries, for industry, science, healthcare professional and patient audiences.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


