Suzanne ElvidgeJuly 15, 2024
Tag: Drug development , Repurposing , Repositioning
Drug development is high risk and lengthy. The wait for potential new treatments is hard for patients, particularly those with rare or hard-to-treat conditions, and if the market is small, the cost of the approved drug may be high. Repurposing and repositioning existing drugs (also known as reprofiling) can speed up the process and cut the required financial investment compared with new drugs, ensuring that patients can get access to treatments more quickly and at more affordable costs. [1, 2]
There are four key groups of repurposed drugs – marketed drugs under existing or expired patents, drugs that have been terminated at clinical or regulatory stages, stereoisomers or metabolites of existing drugs, or candidates where minor changes have been made to existing drugs. It’s important to remember that drug failure in clinical trials isn’t just caused by efficacy or safety issues – it can be because a company changes direction, problems with formulation, or because there have been issues with commercial interest or poor strategic planning. [3]
While the two terms are often used interchangeably, drug repurposing can refer to taking an approved drug and using it for another indication. Drug repositioning can refer to restarting development of a drug that stalled, to gain approval for a new indication. [1]
The primary benefits of repurposing or repositioning drugs are the cost and time aspect. The time frame for developing a repurposed drug is typically one to three years, compared with an average of 12 years for a novel drug. [4]
For a company developing a drug that has stalled in development, or one that has reached the market for another indication, the ability to ‘recycle’ the existing preclinical and/or clinical safety, toxicity and pharmacokinetics/pharmacodynamics data will save both money and time. It also allows them to recoup the money invested in what might otherwise be a failed drug. The existing knowledge also reduces the risk of failure.
Drugs that have failed or are failing in development but still have some patent protection may be repositioned for another indication by the originator company or a licensee. Repurposing can also allow companies to extend the lifecycle of their own marketed drugs that are approaching patent expiration, as they can gain protection for the new indication. Repurposing and repositioning drugs can also be valuable for companies developing drugs for rare diseases, by encouraging collaboration, and the sharing of data and resources. [3, 5]
The steps for developing and gaining approval for a repurposed drug can include:
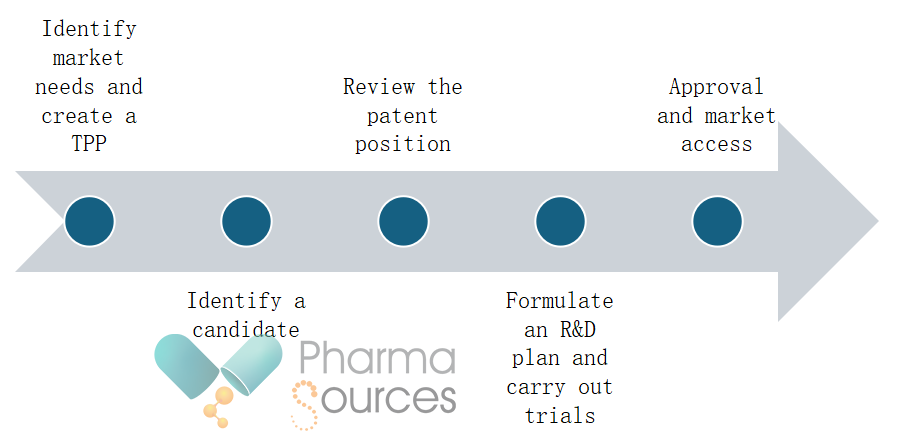
For a company that wants to repurpose a drug but doesn’t have an existing candidate in mind, the first step is to evaluate the market landscape to see whether there is a patient need for a new drug and whether the company will be able to recoup the investment in drug development. This is supported by developing a target product profile (TPP). The TPP describes the desired characteristics of a drug in development for a specific disorder. It provides the company with a guide throughout drug development for all the teams involved. [2, 6, 7]
Areas covered in a TPP, which are also useful to review when a company has a specific candidate in mind, include: [2, 7, 8]
●Indication
○Initial target indication
○Potential future indications
●Population
○Initial target market – economic priorities or areas of greatest unmet need
○Study feasibility
○Requirement for a companion diagnostic to identify sub-populations
●Targeting/pharmacokinetics
●Safety, tolerability and efficacy
○Study endpoints
○Benefits compared with current/future competitors or standard of care
●Drug-drug interactions
●Stability
○Requirements for storage
○May depend on target markets
●Route of administration/formulation
○Current formulation/potential for reformulation
○Which route of administration will be preferred by patients?
○Will a delivery device be required and is it already available
●Dosing frequency
○Consider in comparison with standard of care
●Cost
○Target cost per dose, based on competitive landscape analysis
●Time to availability
○Time required for clinical trials and regulatory processes
○Time required for health technology assessments (HTAs)
A company may have a drug in mind as a repurposing candidate, for example basing a new indication on a finding seen as a side effect in a prior development process.

For a company seeking a drug for a specific target or indication, approaches can be target–, structure–, signature–, pathway–, network–, knowledge– or clinical data–based, with specific approaches including: [13-17]
● Mendelian randomization to establish relationships between phenotypes and genetically predicted drug effects
● Large-scale multi-omics data to better understand disease aetiology and identify novel drug targets
● Machine learning to identify disease subtypes and drug targets, or to link pathology and molecular mechanisms
●Generative AI or integrating human gene expression, drug perturbation, and clinical data, to identify candidates
Review the patent position
A company wanting to repurpose or reposition a drug needs to be aware of the existing patent position, including patents for the original compound, and for formulations, dosage regimens or specific uses. If the drug is still covered by a patent, then they will need to contact the patent holder and see if they are interested in collaborating or licensing out the drug. If the drug is off-patent, there is greater freedom to operate. [4]
All drugs, including repurposed and repositioned drugs, need a level of patent protection in order to allow the repurposing company to recoup its costs before facing competition. New patent types could include: [4, 18]
●Therapeutic indication
●Formulation
●Dose regimen
● Delivery system
● Combination of drugs
●Drug/device combination
To successfully develop a repurposed drug, companies need to work with clinicians and a multidisciplinary team of internal and external experts to support the development process. Repurposed drugs still have to have sufficient preclinical data, go through clinical trials and meet exacting regulatory standards in their new indication. While existing human and non-human data will support safety and toxicity, further clinical studies will be needed to confirm efficacy in the target patient group before submitting for approval. [8] Working with patients and patient advocacy groups is also important, to understand what they need from a treatment, and to recruit participants for clinical trials. [4]
Close collaboration with the regulatory authorities and health technology assessment (HTA) bodies will support an efficient process towards approval, market and reimbursement. Regulatory authorities can advise what clinical trials will be required, and suggest which route to approval, including expedited routes, will be the most appropriate for a specific drug and indication. [4]
Drug repurposing and repositioning offers a cost effective and efficient route to the market that allows patients to get access to drugs more quickly. Companies need to be sure that they collect the right data for a smooth process, and this can be helped by collaborating with patients, other companies, regulatory authorities and health technology assessment bodies.
1. Bakker, A., A seemingly small semantic issue is a major roadblock to develop treatments for rare diseases. STAT, 2023. Available from: https://www.statnews.com/2023/06/27/drug-repurposing-repositioning-rare-diseases/.
2. Griffiths, A., et al., Target Product Profiles in Pharmaceutical Development KPMG. 2023. Available from: https://assets.kpmg.com/content/dam/kpmg/uk/pdf/2023/01/target-product-profiles-in-pharmaceutical-development.pdf.
3. Elvidge, S., Getting The Drug Repositioning Genie Out Of The Bottle. Life Science Leader, 2010. Available from: https://www.lifescienceleader.com/doc/getting-the-drug-repositioning-genie-out-of-the-bottle-0001.
4. Pisani, J., et al., Repurposing medicines: the opportunity and the challenge. 2021. Available from: https://www.lifearc.org/wp-content/uploads/2024/03/RD-Drug-repurposing-report.pdf.
5. Taylor, M., M. Salova, and A. Schroeder, Drug Repurposing: Potential to Expand Rare Disease Treatment. Avalere: Insights & Analysis, 19 February 2024. Available from: https://avalere.com/insights/drug-repurposing-potential-to-expand-rare-disease-treatment.
6. Staff writer. Target product profiles. World Health Organization. 9 July 2024. Available from: https://www.who.int/observatories/global-observatory-on-health-research-and-development/analyses-and-syntheses/target-product-profile/who-target-product-profiles.
7. Staff writer. Repurposing of a drug: key considerations. UCL Therapeutic Innovation Networks - UCL – University College London. 9 July 2024. Available from: https://www.ucl.ac.uk/ion/translation-enterprise/tailored-support-translational-researchers/re-purposing-drug/repurposing-drug.
8. Staff writer. Repurposing Medicines Toolkit - Guidance for navigating the process. LifeArc/Medical Research Council. 11 July 2024. Available from: https://www.repurposingmedicines.org.uk.
9. Roundtable on Translating Genomic-Based Research for Health, Board on Health Sciences Policy, and Institute of Medicine, in Drug Repurposing and Repositioning: Workshop Summary. 2014: Washington (DC).
10. Ghofrani, H.A., I.H. Osterloh, and F. Grimminger, Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov, 2006. 5(8): p. 689-702.
11. Gohel, D., et al., Sildenafil as a Candidate Drug for Alzheimer's Disease: Real-World Patient Data Observation and Mechanistic Observations from Patient-Induced Pluripotent Stem Cell-Derived Neurons. J Alzheimers Dis, 2024. 98(2): p. 643-657.
12. Dorset Medicines Advisory Group, SHARED CARE GUIDELINE FOR SILDENAFIL FOR THE MANAGEMENT OF SECONDARY RAYNAUDS PHENOMENON ASSOCIATED WITH SYSTEMIC SCLEROSIS NHA. 2017. Available from: https://nhsdorset.nhs.uk/Downloads/aboutus/medicines-management/Shared%20Care%20Guidelines/Sildenafil%20Shared%20Care%20Documented%20July%2017.pdf.
13. Wang, L., et al., The landscape of the methodology in drug repurposing using human genomic data: a systematic review. Brief Bioinform, 2024. 25(2).
14. Sperry, M. and D.E. Ingber, Drug Repurposing Strategies, Challenges and Successes. 4 March 2024. Available from: https://www.technologynetworks.com/drug-discovery/articles/drug-repurposing-strategies-challenges-and-successes-384263#D3.
15. Rodriguez, S., et al., Machine learning identifies candidates for drug repurposing in Alzheimer's disease. Nat Commun, 2021. 12(1): p. 1033.
16. Yan, C., et al., Leveraging generative AI to prioritize drug repurposing candidates for Alzheimer's disease with real-world clinical validation. NPJ Digit Med, 2024. 7(1): p. 46.
17. Wu, P., et al., Integrating gene expression and clinical data to identify drug repurposing candidates for hyperlipidemia and hypertension. Nat Commun, 2022. 13(1): p. 46.
18. Conour, J., Why Method of Treatment Patents for Repurposed Drugs Are Worth the Investment. JD Supra: News & Insights, 5 October 2020. Available from: https://www.jdsupra.com/legalnews/why-method-of-treatment-patents-for-92813/.
Based in the north of England, Suzanne Elvidge is a freelance medical writer with a 30-year experience in journalism, feature writing, publishing, communications and PR. She has written features and news for a range of publications, including BioPharma Dive, Pharmaceutical Journal, Nature Biotechnology, Nature BioPharma Dealmakers, Nature InsideView and other Nature publications, to name just a few. She has also written in-depth reports and ebooks on a range of industry and disease topics for FirstWord, PharmaSources, and FierceMarkets. Suzanne became a freelancer in 2006, and she writes about pharmaceuticals, consumer healthcare and medicine, and the healthcare, pharmaceutical and biotechnology industries, for industry, science, healthcare professional and patient audiences.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


