Saher HaiderJuly 02, 2024
Tag: Pharmaceutical drug policy , healthcare , regulatory
Pharmaceutical products are the heart of the healthcare system, yet their accessibility remains a challenge for people across the globe. Issues like scarcity, high costs, and insufficiently trained health workers prevent people from accessing vital medicines needed for the prevention and treatment of several illnesses.
This is where the Pharmaceutical Drug Policy comes in.
Pharmaceutical policy is an integral component of health policy dealing with the development, supply, and use of medicines in a healthcare system. The main goal of having stringent pharmaceutical policies in place is to provide access to safe, efficacious, and affordable medicines to everyone.
However, it is easier said than done. Despite having innumerable advantages of maintaining drug policies, there are many setbacks when it comes to creating and implementing these, with the main one being the financial interests of suppliers and healthcare providers. Since both suppliers and healthcare providers benefit from charging higher prices and issuing unnecessarily long prescriptions, it is crucial to regulate these for the welfare of patients.
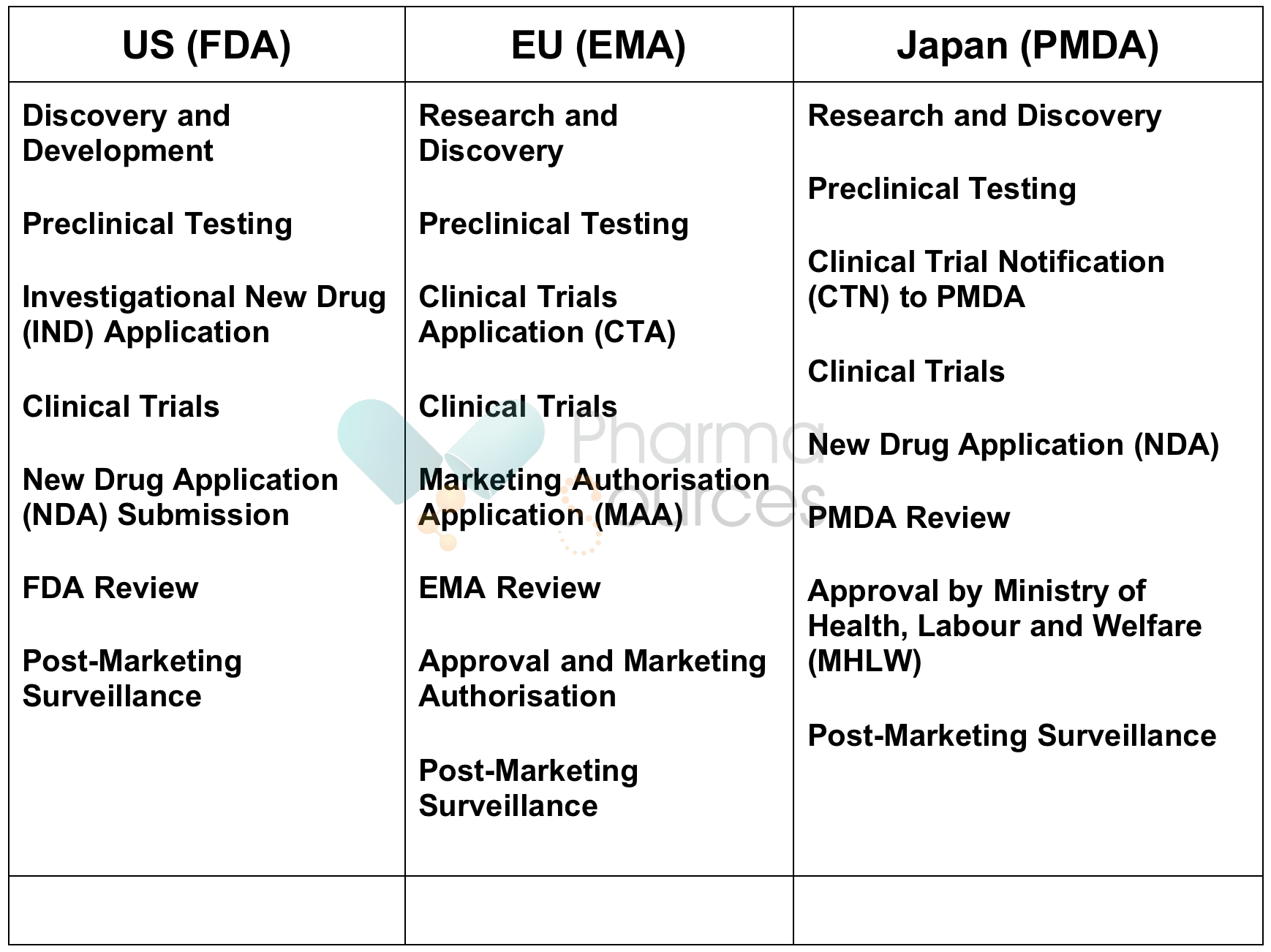
Regulatory agencies oversee pharmaceutical organizations within their respective jurisdictions, ensuring compliance with safety, efficacy, and quality standards. The Food and Drug Administration (FDA) in the United States, the European Medicines Agency (EMA) in the European Union, and the Pharmaceuticals and Medical Devices Agency (PMDA) in Japan are notable examples of globally renowned regulatory agencies. While the specific structure and responsibilities of these agencies may vary, they all are responsible for evaluating drug applications, providing marketing approvals, conducting inspections of manufacturing facilities, and monitoring adverse drug reactions.
Since pharmaceutical industries are the most regulated industries in the world, regulatory agencies are primarily responsible for assuring the safety and efficacy of pharmaceutical products before they reach patients. Through rigorous review processes, these agencies assess preclinical and clinical data to determine whether a drug's benefits outweigh its risks. They also provide guidelines for setting and enforcing standards for manufacturing practices, labeling requirements, and advertising regulations.
Despite shared goals of ensuring patient safety and therapeutic effectiveness, drug approval processes vary across countries. These variations are vital as disparities influence access to medicines, patient outcomes, and the efficiency of healthcare systems.
Differences in legal requirements, resource availability, and risk tolerance are key reasons behind variations in drug approval processes. For instance, some regulatory agencies prioritize expedited review pathways for innovative therapies addressing unmet medical needs, while others adhere to more stringent review timelines. Differences in data requirements, clinical trial standards, and post-marketing surveillance practices also contribute to variations in approval processes.
Below is the comparative analysis of drug approval processes in the US, EU, and Japan:
Several factors influence access to pharmaceutical drugs, including healthcare coverage, reimbursement policies, and pricing regulations. In countries with robust universal healthcare systems, such as those with single-payer models, access to essential medicines is ensured through comprehensive coverage mechanisms. Conversely, in nations relying on private insurance models or out-of-pocket payments, access may be contingent upon individuals' ability to afford healthcare services. Pricing regulations, including government negotiations with pharmaceutical companies and price controls, also determine drug affordability and accessibility.
Let’s take an example of pricing structures in the US vs Japan to understand how access to pharmaceutical drugs varies from country to country.
The Japanese healthcare system, overseen by the Ministry of Health, Labour and Welfare, uses a multi-payer public insurance model. In this model, prices for medicines are determined through Ministry procedures.
For drugs with alternatives, the new medicine’s daily price matches that of the comparable drug, ensuring fair competition. For unique drugs, the price is based on production costs, R&D, marketing expenses, and a fixed profit margin. Prices are also adjusted based on average prices in the US, UK, Germany, and France. For example, prices above 25% of the average are reduced, and those below are increased. Generic drugs are priced at 50% of the original, or 40% if more than ten brands exist.
Due to rising costs from an aging population and new expensive technologies, a cost-effectiveness analysis system was introduced in 2019. Drugs not meeting defined cost-effectiveness criteria have their prices adjusted based on incremental cost-effectiveness ratios (ICERs). If an ICER is below ¥5 million ($43,800) per Quality Adjusted Life Year (QALY), the price remains unchanged; otherwise, prices are adjusted downward.
The US pharmaceutical pricing system differs significantly, emphasizing competition over regulation. Unlike other countries where the government sets prices or negotiates with manufacturers, the US prohibits federal negotiation and relies on market competition. This policy was solidified with the Hatch-Waxman Act of 1984.
Government programs like Medicare Part D and Medicaid support over 40% of retail prescriptions, but private insurers, often funded through employee benefit packages, cover most prescriptions. This system benefits from tax advantages, making private insurers the primary purchasers of pharmaceuticals.
Universal healthcare systems, characterized by government-funded healthcare coverage for all residents provide equitable access to essential services, including pharmaceutical drugs. In contrast, private insurance models rely on market-based mechanisms, where individuals purchase insurance plans to access healthcare services, including prescription medications.
While universal healthcare systems offer comprehensive coverage and mitigate financial barriers to access, private insurance models may offer greater flexibility and choice but can result in disparities in access based on socioeconomic status.
Let us take an example from healthcare systems in the US, EU, and Japan to do a comparative analysis of universal healthcare system vs private insurance models.
Japan’s healthcare system is a universal multi-payer model, where all residents are covered under public insurance schemes. The government regulates and sets drug prices, ensuring equitable access to pharmaceuticals. Patients pay a percentage of their medical costs, with the rest covered by insurance. This universal multi-payer model ensures that all citizens get access to necessary medications without a significant financial burden.
Many EU countries, such as the UK and Germany, operate under universal healthcare systems. The UK’s National Health Service (NHS) provides comprehensive healthcare services, including pharmaceuticals, funded through taxation. Germany uses a statutory health insurance system where public insurance covers the majority of healthcare costs, and private insurance plays a supplemental role. In both countries, drug prices are regulated to ensure affordability and access for all residents.
Pros of Universal Healthcare Systems
· Equitable access to healthcare services, including essential medications.
· Reduced financial barriers to accessing necessary treatments.
· Government-regulated prices help control pharmaceutical costs.
Limitations
· Longer wait times for certain treatments.
· Limited flexibility and choice compared to private insurance models.
The US healthcare system primarily relies on private insurance, where individuals purchase insurance plans to access healthcare services. Medicare and Medicaid provide coverage for specific populations (seniors, and low-income individuals), but the majority of healthcare is funded through employer-sponsored insurance plans or private purchases. Drug prices are not regulated by the government, leading to higher costs and potential disparities in access based on socioeconomic status.
Pros of Universal Healthcare Systems
· Greater flexibility and choice in selecting healthcare providers and treatments.
· Innovation is driven by market competition.
Limitations
· Significant disparities in access based on income and employment status.
· High out-of-pocket costs and potential financial hardship for uninsured or underinsured individuals.
Comparative Analysis
Universal healthcare systems, such as those in Japan and many EU countries, ensure that all residents have access to essential healthcare services, including pharmaceuticals, through government-funded programs. These systems provide equitable access and mitigate financial barriers but may offer less flexibility and longer wait times.
In contrast, the US private insurance model offers more flexibility and choice but can lead to significant disparities in access and higher costs for individuals. Balancing the strengths and limitations of each model can help policymakers design systems that optimize access to pharmaceutical drugs while ensuring financial sustainability and equity.
Generic substitution is a key strategy for reducing healthcare costs and increasing access to medications. Generic drugs help alleviate financial burdens on patients and healthcare systems by offering therapeutically equivalent alternatives to brand-name drugs at lower prices.s.In the US, the FDA supports this by encouraging the use of generics through educational campaigns and incentives. Similarly, in the EU, the European Medicines Agency (EMA) promotes the use of generic medicines to enhance cost savings and equitable access to essential medications. Japan also adopts measures to encourage the use of generics, including price adjustments and educational initiatives
Bioequivalence testing is an essential part of generic drug substitution. It ensures that generic drugs perform similarly to their brand-name counterparts in terms of safety, efficacy, and pharmacokinetic properties. This is why regulatory agencies impose stringent bioequivalence requirements to guarantee the therapeutic equivalence of generic drugs, thus safeguarding patient health and confidence in generic products.
Pharmaceutical drug policy is an essential component of health policy dealing with the development, supply, and use of medicines in a healthcare system. In this article, we reviewed two drug policies in the three biggest pharmaceutical markets in the world - the US, Japan, and the EU. While the regulatory approval processes are similar in these regions, it’s the healthcare system regulating access to medicinal products that varies significantly between the US and Japan/EU.
Saher Binte Haider is a pharmacy graduate from Dow University of Health Sciences. She started her career as a Quality Management professional in the pharmaceutical industry where she developed a keen interest in good documentation practices, SOP creation, and content writing. She has 7+ years of experience in healthcare & life sciences content writing. Her key areas of expertise are healthcare, pharmaceuticals, health tech, and AI in healthcare.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


