Far-away from the CrowdMay 06, 2024
Tag: ROR1 Target , ADC , Anti-tumor
On April 3, it was jointly announced that the two companies, Ipsen and Sutro Biopharma, had reached a global exclusive licensing agreement totaling up to USD 900 million for Sutro's antibody-drug conjugate (ADC) STRO-003. STRO-003 will be the first ADC candidate drug in Ipsen's portfolios.
STRO-003 is a new-generation ROR1-targeted ADC, which could generate highly stable conjugates with exatecan payloads by utilizing Sutro's site-specific technology. The strong single-drug efficacy and differentiated safety that STRO-003 shown in preclinical trials of both solid tumors and hematological malignant tumors has given it the potential to be "best-in-class" drugs.
According to the terms of the agreement, Ipsen will prepare for the STRO-003 Phase I clinical trial, including the submission of an IND application, as well as all subsequent clinical development and global commercialization activities. Sutro is eligible to receive potential upfront, developmental, regulatory, and commercial milestone payments totaling up to USD 900 million, including an immediate payment of around USD 90 million, contingent upon the successful development and commercialization of the drug.
ROR1, short for receptor tyrosine kinase-like orphan receptor 1, is a kind of transmembrane protein in ROR receptor family. In recent years, due to its high expression characteristics in various malignant tumors, ROR1 has become a research hotspot in biomedicine and is considered to be a new drug target with broad-spectrum anticancer potential.
Unlike less-expressed or non-expressed in normal human tissues, ROR1 is highly expressed in many malignant tumors or tissues. Take diseases such as chronic lymphocytic leukemia (CLL), breast cancer, ovarian cancer, melanoma, and lung adenocarcinoma for example, the characteristics of high expression in tumors but low expression in healthy cells make ROR1 an attractive drug R&D objective. After binding to ligand Wnt5a, ROR1 is able to mediate the signaling of non-classical Wnt signaling pathway, which will play an important role in promoting tumor growth and metastasis, inducing drug resistance and inhibiting apoptosis of tumor cells.
Southwest Securities Research Report predicts that the market is expected to obtain tens of billions of dollars once ROR1 ADC is developed into a drug. Such a huge market size has naturally attracted many MNCs' attention. In the short span of three months from October to December 2020, CSTONE Pharmaceuticals, MSD, and Boehringer Ingelheim made significant investments to acquire three kinds of ROR1 ADCs. CSTONE Pharmaceuticals introduced the ROR1-targeted ADC drug CS5001 that developed by the Korean biopharmaceutical company LCB with USD 10 million advance payments and up to USD 353 million milestone payments and additional tiered royalties. MSD spent USD 2.75 billion to acquire VelosBio, whose core pipeline is a kind of ROR1 ADC drug, called zilovertamab vedotin; Boehringer Ingelheim spent EUR 1.18 billion to acquire NBE-Therapeutics, focusing on acquiring a kind of ROR1 ADC drug, called NBE-002. There are only three kinds of ROR1 ADCs that have entered the clinical stage in the world at present.
Zilovertamab vedotin (MK-2104) is composed of a monoclonal antibody targeting ROR1 connected to the chemotherapeutic agent MMAE. The combination of the antibody to MK-2140 and the ROR1 on cancer cells can release MMAE to destroy cancer cells. VLS-101 has shown great anti-tumor efficacy in mouse models of human hematological malignant tumors and solid tumors. Previously, the FDA granted VLS-101 orphan drug designation and fast track status for the treatment of Mantle Cell Lymphoma (MCL).
At the European Society for Medical Oncology in 2021 (ESMO 2021), the escalation result of the first-in-human dose in Phase I patients that MK-2140 used in treatment of malignant tumor lymphatic system were announced. Data indicated that the candidate drug induced objective tumor responses in 7 out of 15 Mantle Cell Lymphoma patients, with an objective response rate (ORR) of 47%, comprising 4 partial responses and 3 complete responses. Among the 5 patients with DLBCL, 3 achieved objective response (ORR of 60%), with 1 partial response and 2 complete response. No ORR was observed in patients with other tumor types.
At the 2023 ASCO, MSD disclosed the Phase II open-label data of MK-2140 for the treatment of terminal line DLBCL and the waveLINE-004 study data. The results showed that the ORR of patients was 30% (6 out of 20 patients) after MK-2140 treatment, with 2 CR and 4 PR. In summary, zilovertamab vedotin has shown a great potential in hematologic tumor treatment of terminal line.
According to the Clinicaltrials.gov official website, MK-2140 is currently conducting multiple clinical research of B-cell lymphoma around the world. Among them, the research (NCT05139017) for B-cell lymphoma (DLBCL) indication Phase II/III, which characterized by multicenter, open-label, randomized and positive-controlled, has been initiated in December 2021 and is estimated to be finished in December 2025, showing the fastest-growing ROR1 ADC currently. In November 2021, the clinical application of MK-2140 was accepted by NMPA in China, which marks the first ROR1 ADC declared for clinical application in China.
NBE-002 is a kind of ROR1-targeted ADC, formed through site-specific enzymatic conjugation of the anthracene-like compound PNU-159682 with an ROR1 antibody via an indivisible linker. NBE-002's direct antitumor activity was evaluated in immunodeficient PDX models representing several tumor and sarcoma subtypes with varying levels of ROR1 expression, ranging from low to high. Remarkably, its most pronounced antitumor effects were observed in triple-negative breast cancer, with efficacy demonstrated at doses as low as 0.033 mpk. NBE-002 is now in Phase I/II that applied in Triple Negative Breast Cancer indication, but it seems that Boehringer Ingelheim have stopped the R&D of the drug. Besides, the NBE-002 is no longer in the R&D pipeline disclosed on its official website.
CS5001 is a kind of ADC composed of a human monoclonal antibody of ROR1. With the technology of directional coupling, CS5001 binds a unique Beta-glucuronide linker and a Pyrrole benzodiazepines (PBD) prototoxin dimer. CS5001 has many differentiated features, including exclusive site-specific coupling, tumor-selective divisible linker and prodrug technology, which has shown its greatest potential of the same type in Mantle Cell Lymphoma and triple-negative breast cancer xenograft models. Its Phase I clinical trial has completed the evaluation of seven dose levels and the encouraging characteristics of safety, stability, and anti-tumor activity have been observed in a variety of malignant hematological and solid tumors. Much more Phase I clinical data are expected to be disclosed at international academic conferences in the first half of this year. In May 2022, the NMPA approved the clinical trial application for CS5001, intended for the development in treating advanced hematologic tumors and solid tumors.
In addition to the above mentioned three ROR1 ADCs in clinical stages, several preclinical ADCs have also demonstrated promise. For instance, STRO-003, recently introduced by Ipsen at a premium, exhibited antitumor activity in preclinical models of NSCLC and breast cancer, with an expected IND application submission in the near future. Moreover, Immunome made a notable appearance at the recent JPM with its core pipeline IM-1021, a ROR1 ADC expected to file for IND in the first quarter of 2025.
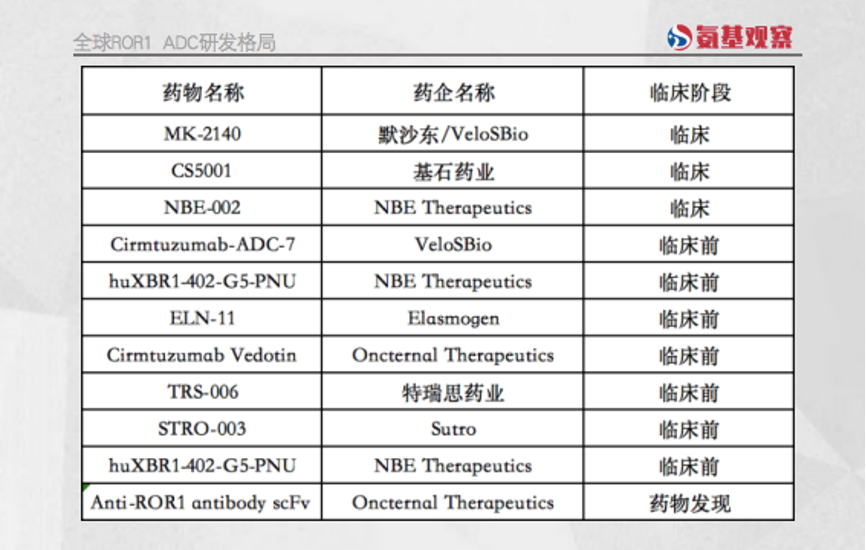
Figure Source: Anji Observation
全球ROR1 ADC研发格局 | Global ROR1 ADC R&D pattern |
氨基观察 | Anji Observation |
药物名称 | Drug names |
药企名称 | Pharmaceutical companies |
临床阶段 | Clinical phase |
默沙东/VeloSBio | MSD/VeloSBio |
基石药业 | CSTONE Pharmaceuticals |
特瑞思药业 | Teruisi |
临床 | Clinical |
临床前 | Preclinical |
药物发现 | Drug discovery |
The reason that ROR1 is a target with great potential and attention is not the anti-tumor efficacy of the ROR1 target itself, but because of its characteristics of high expression in tumor cells but low expression in adult healthy tissues. Such a kind of target is the most ideal ADC drug target. The large patient population, high tumor-specific targets and therapeutic vacancies endow ROR1 ADC with a great room for imagination. At present, ROR1 ADC has shown good therapeutic effects in terminal line hematologic tumors and triple-negative breast cancer. We look forward to more ROR1 ADCs producing strong clinical data and achieving major breakthroughs in the treatment of solid tumors and hematologic tumors.
1. Official websites of each enterprise
2. Baskar S, K wong KY, .Hofer T, et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia [J]. Clin Cancer Res. 2008, 14:396-404.
3. Zhao Yuming, Zhang Dengyang, Guo Yao et al. Tyrosine Kinase ROR1 as a Target for Anti-Cancer Therapies. [J] .Front Oncol, 2021, 11: 680834.
4. Vaisitti Tiziana, Arruga Francesca, Vitale Nicoletta et al. ROR1 targeting with the antibody-drug conjugate VLS-101 is effective in Richter syndrome patient-derived xenograft mouse models. [J] .Blood, 2021, 137


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


