Shruti TalashiMarch 08, 2024
Tag: Microbiome , Fecal matter transplantation , Bioactives
To gain a better knowledge of the micro-biome's role in health and disease, the human microbiome project was started in 2007. With a deluge of published research during the next 20 years, our knowledge of the microbiome's composition and its relationship to human health and disease has rapidly increased. A break in this symbiotic interaction has been linked to a number of disorders related to the microbiome. Growing research indicates that the microbiome affects cancer, neurological illnesses, and autoimmune diseases. [1]
Microbiome-based therapies are currently gaining popularity for treating a wide range of illnesses, such as infections, inflammatory conditions (IBDs, atopic dermatitis), neurological conditions (Parkinson's disease, autism), cancer, and so on. A major victory that inspires excitement in the sector is the FDA's approval of Ferring's REBOTYA & SER-109 as the first microbiome therapy for patients with recurrent C. diff infections. The methods for modifying the microbiome to enhance human health and treat illnesses have changed over time, and they differ substantially depending on the mechanism of action and kind of asset, such as ecological, donor-derived, and donor-independent methods. The formal classification of microbiome therapies is still up for debate because the area is constantly developing. [2]
Products based on fecal matter transplantation (FMT) allow the patient to get the entire gut microbial ecology from a donor. Such complete microbiomes or donor derived microbiome asset involve the isolation and subsequent transfer of a donor's uncharacterized microbiome. The microbial population is isolated from cell banks in a broad consortium which is donor independent approaches that may have a donor origin and were later purified to be used as a pharmaceutical. More specifically designed methods, such as narrow and defined consortia, include combining various strains to affect a patient's microbiome. While defined consortia are donor independent and rely on cell banks, narrow consortia depend on sample donors. In some situations, a single strain of bacteria may be adequate and productive. These naturally occurring single strain products are referred to as engineered single strains when they undergo additional genetic modification for a better mode of action. Bacteriophages are viruses that infect bacteria and are capable of eliminating particular strains of bacteria, such as those that cause disease in the microbiome. Phage therapy may also prove to be highly effective in combating "superbugs" that are resistant to antibiotics. Such lytic phages are incapable of dispersing resistance or virulence since they kill bacteria. [3]
Bioactives directly modify the microbiome in order to promote health. These bioactives are pre-biotics help the good bacteria in the stomach to develop, and post-biotics that are byproducts of the metabolism of probiotics and prebiotics. Post-biotics can include basic short-chain fatty acids like butyric acid, which can support a healthy microbiome, or specific vitamins, amino acids, and peptides with antibacterial qualities. The most well-defined technique is using small compounds to directly alter the microbiome, but doing so also necessitates a thorough comprehension of their mode of action.[4]
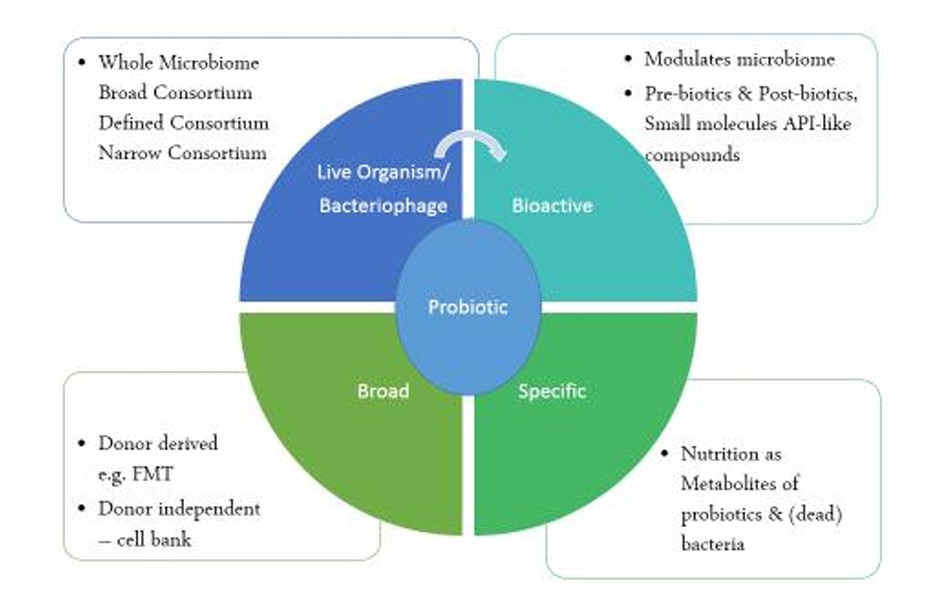
Figure above shows spectrum of microbiome therapeutics where microbiome assets is classified in donor-derived, donor-independent and from ecological towards more defined approaches
The global market for microbiome therapeutics is estimated to increase from $164.8 million in 2022 to reach $1.5 billion by 2027, at a compound annual growth rate (CAGR) of 54.8% from 2022 through 2027.
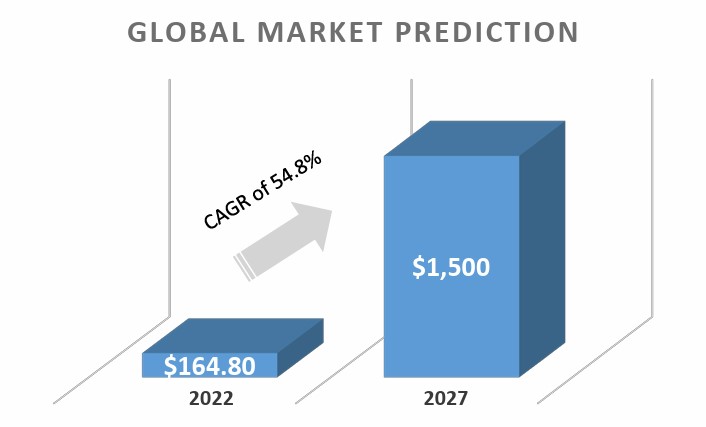
Figure above forecast for global microbiome therapeutics market in USD million over the next few years (2022-2027).
Probiotics are a subset of single strain applications that are mostly nutritional items containing live microorganisms used to enhance gut health. The probiotics marker is predicted to grow at a CGAR of 8.1% between 2022 and 2027 reaching value of USD 85.4 billion projection by 2027 from being at USD 57.8 billion in 2022 while Asia-Pacific had dominated the probiotic market.[5]
Certain firms are using checkpoint inhibitors to treat cancers by aiming to stimulate the immune system. A medication and microbe combination has been created by Vedanta Biosciences to stimulate helper T-cells against cancers and is currently in phase 1 trial. Atopic dermatitis is treated by 4D Pharma using single microbes for immunomodulation from development code B244, while Evelo Biosciences is also developing the single-microbiome technique. The physiologically active chemicals of bacteria are the focus of Second Genome, Kaleido Biosciences, and Enterome. Enterome has created a medicine that is presently undergoing a phase 2 study by focusing on the signaling system that causes Crohn's disease. A microbial vaccine is currently being developed by 4D Pharma and Merck. We anticipate that more medicines will be approved for sale in the future, and that this initial discovery will herald a new age in microbiome treatments.[6]
In conclusion, globally human microbiome therapies are both a developing field with enormous potential in healthcare and a market that is growing quickly. The success of early treatments and our growing understanding of the role the microbiome plays in a variety of diseases point to a bright future for this research. Microbiome medicines are now being investigated for a variety of conditions, including atopic dermatitis, cancer, inflammatory bowel disease, and the creation of microbial vaccines.
Saura C. Sahu & A. Wallace Hayes, The Human Microbiome: History and Future, J Pharm Pharm Sci (www.cspsCanada.org) 23, 406 - 411, 2020.
Hitch, T.C.A., Hall, L.J., Walsh, S.K. et al. Microbiome-based interventions to modulate gut ecology and the immune system. Mucosal Immunol 15, 1095–1113 (2022). https://doi.org/10.1038/s41385-022-00564-1.
Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol. 2016 Mar;9(2):229-39. doi: 10.1177/1756283X15607414. PMID: 26929784; PMCID: PMC4749851.
Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014 May 5;11(5):4745-67. doi: 10.3390/ijerph110504745. PMID: 24859749; PMCID: PMC4053917.
Microbial Products: Technologies, Applications and Global Markets; BIO086E BCC Publishing published date Jun 2023, access date Mar 2024.
Ms. Shruti Talashi boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


