zhulikou431March 06, 2024
Tag: Innovative drugs , FDA , Approval and Withdrawal
The R&D of innovative drugs is characterized within the sector as an odyssey "towards vitality from the verge of demise," attributable to the substantial financial requisites, protracted development durations, and elevated incidence of failure. The minority that secure endorsement from global regulatory bodies indeed epitomize rarity. Nonetheless, certain entities, despite securing initial authorization, may witness their approvals rescinded if their post-market performance is suboptimal, if they fail to fulfill the regulatory mandates for subsequent research undertakings, or if the research outcomes are deemed deficient. This document endeavors to compile and elucidate data pertaining to instances where the U.S. FDA, in recent years, has rescinded market approval for pharmaceuticals previously sanctioned.
On February 23, 2024, the FDA disseminated its conclusive verdict to retract the authorization of Pepaxto (melphalan flufenamide). This pronouncement, articulated by Peter Marks, the Director of the FDA's CBER, signifies the inaugural application of the 2023 amended accelerated approval revocation process, as stipulated in the Food and Drug Omnibus Reform Act of 2022 (FDORA). The rationale for the market withdrawal of Pepaxto encompasses: (1) the failure of the confirmatory study, mandated as a condition of the accelerated approval, to corroborate Pepaxto's clinical advantage, and (2) the prevailing evidence indicating that Pepaxto has not been substantiated as safe or efficacious under its prescribed conditions of utilization (Figure 1).
Pepaxto, a product of Oncopeptides' innovation targeting aminopeptidase and heralded as the FDA's inaugural endorsement of an anticancer peptide-drug conjugate (PDC), was accorded accelerated approval on February 26, 2021. It was intended for usage in tandem with dexamethasone for the treatment of patients with relapsed or refractory multiple myeloma (R/R MM). Concurrent with its approval, the FDA mandated Oncopeptides to execute a confirmatory Phase III OCEAN trial. Subsequent to this, the OCEAN trial elucidated disheartening findings, not achieving the clinical endpoint for overall survival (OS) and recording a higher mortality rate in the Pepaxto treatment group as opposed to the control group (overall survival hazard ratio of 1.104). Consequently, the FDA suspended the trial of Pepaxto, issued a safety notification, and resolved to convene a session of the Oncologic Drugs Advisory Committee (ODAC) on October 28, 2021, for the evaluation of Pepaxto's safety profile.
Prior to the assembly of the FDA's expert panel, Oncopeptides announced on October 22, 2021, its volitional withdrawal of Pepaxto from the market. Nevertheless, in January 2022, Oncopeptides retracted its market withdrawal application for Pepaxto, with intentions to "engage in close dialogue with the FDA and reassess the clinical data." Despite these efforts, the American regulatory authority did not align with Oncopeptides' renewed analysis, maintaining that Pepaxto required further evaluation. On July 20, 2022, the FDA once more convened the ODAC to deliberate on the benefit/risk assessment of Pepaxto.
Adhering to the advisory panel's counsel, the FDA officially decreed the revocation of accelerated approval for Pepaxto on December 7, 2022, thus forbidding its commercial distribution. In August 2023, Oncopeptides lodged an appeal against the FDA's decision to withdraw. The decree issued on February 23 is the FDA's response to this appeal. In accordance with the revised protocols for accelerated approval revocation, the FDA issued to Oncopeptides a notice of intended withdrawal, an explanation thereof, an opportunity for a hearing, and a provision for submitting a written appeal to the Commissioner (or an appointed delegate). Following the submission of a written appeal, Oncopeptides engaged in a consultation with an appointed delegate of the Commissioner.
The Accelerated Approval (AA) protocol was inaugurated by the FDA in 1992, primarily to address the exigencies of the HIV/AIDS pandemic. Post its codification within the Food and Drug Administration Safety and Innovation Act (FDASIA) in 2012, the issuance of accelerated approvals experienced a notable surge, especially within the antitumor drug sector (Figure 2).
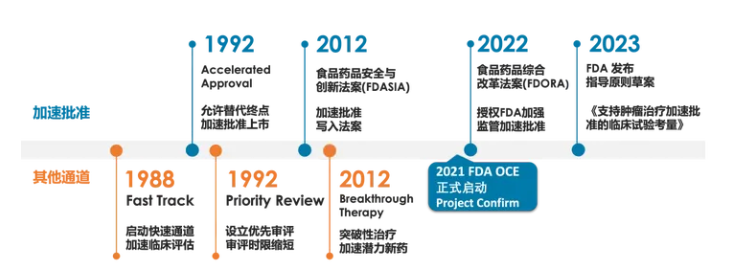
Figure 2. The Transformation of FDA's Accelerated Approval Process
In comparison with the conventional approval framework, the Accelerated Approval (AA) mechanism abbreviates the median market introduction timeline for pharmaceuticals by 3.1 years. Consequently, it has emerged as a significant conduit for the commercialization of numerous new oncology medications. Nevertheless, the execution of this expedited pathway has elicited considerable debate, centering on three pivotal concerns: the substantiation of clinical benefits derived from surrogate endpoints, the scheduling of confirmatory studies, and the retraction of market authorization for drugs failing validation.
As of the present, the FDA has rescinded accelerated approval for a collective total of 27 drugs. A compendium of these instances is presented herein for professional perusal and consideration.
Drug Name | Accelerated Approval Indication | Accelerated Approval Date | Withdrawal Date |
Pepaxto (melphalan flufenamide) | In combination with dexamethasone for the treatment of triple-class refractory/relapsed multiple myeloma (R/R MM) | February 26, 2021 | February 23, 2024 |
Ukoniq (umbralisib) | For adult patients with relapsed or refractory marginal zone lymphoma (MZL) who have received at least one prior anti-CD20-based regimen | February 5, 2021 | May 31, 2022 |
Ukoniq (umbralisib) | For adult patients with relapsed or refractory follicular lymphoma (FL) who have received at least three prior systemic therapies | February 5, 2021 | May 31, 2022 |
Gavreto (pralsetinib) | For the treatment of adult and pediatric patients 12 years of age and older with advanced or metastatic RET mutant medullary thyroid cancer (MTC) requiring systemic treatment | December 1, 2020 | July 20, 2023 |
Blenrep (belantamab mafodotin-blmf) | For adult patients with relapsed or refractory (R/R) multiple myeloma (MM) who have received at least four prior therapies including an anti-CD38 monoclonal antibody, a proteasome inhibitor, and an immunomodulatory agent | August 5, 2020 | February 6, 2023 |
Keytruda (pembrolizumab) | For patients with metastatic small cell lung carcinoma (SCLC) that has progressed on or after platinum-based chemotherapy and at least one other prior line of therapy | June 17, 2019 | March 30, 2021 |
Tecentriq (atezolizumab) | In combination with paclitaxel protein-bound for the treatment of unresectable locally advanced or metastatic triple-negative breast cancer (TNBC) expressing PD-L1 as determined by an FDA-approved test, with tumor-infiltrating immune cells covering ≥1% of the tumor area | March 8, 2019 | October 6, 2021 |
Copiktra (duvelisib) | For the treatment of adult patients with relapsed or refractory follicular lymphoma (FL) after at least two prior systemic treatment | September 24, 2018 | December 17, 2021 |
Opdivo (nivolumab) | For patients with metastatic small cell lung carcinoma (SCLC) that has progressed after platinum-based chemotherapy and at least one other line of therapy | August 16, 2018 | December 29, 2020 |
Keytruda (pembrolizumab) | For the treatment of recurrent, locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma patients whose tumors express PD-L1 (CPS≥1) as determined by an FDA-approved test, after two or more previous lines of therapy including fluoropyrimidine- and platinum-containing chemotherapy and if applicable, HER2/neu-targeted therapy | September 22, 2017 | February 4, 2022 |
Opdivo (nivolumab) | For patients with hepatocellular carcinoma who have been previously treated with sorafenib | September 22, 2017 | July 23, 2021 |
Imfinzi (durvalumab) | For patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy | May 1, 2017 | February 19, 2021 |
Tecentriq (atezolizumab) | For patients with locally advanced or metastatic urothelial carcinoma (mUC) who are not eligible for cisplatin-containing chemotherapy and whose tumors express PD-L1 as determined by an FDA-approved test, or in patients who are not eligible for any platinum-containing chemotherapy regardless of PD-L1 status | April 17, 2017 | December 2, 2022 |
Imbruvica (ibrutinib) | For the treatment of adult patients with marginal zone lymphoma (MZL) who require systemic treatment and have received at least one prior anti-CD20-based therapy | January 18, 2017 | May 18, 2023 |
Lartruvo (olaratumab) | In combination with doxorubicin for the treatment of adult patients with soft tissue sarcoma (STS) that is histologically subtype suitable for an anthracycline-containing regimen and is not amenable to radiotherapy or surgery. | October 19, 2016 | February 25, 2020 |
Tecentriq (atezolizumab) | For patients with locally advanced or metastatic urothelial carcinoma who have disease progression during or following platinum-containing chemotherapy, or within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy | May 18, 2016 | April 13, 2021 |
Farydak (panobinostat) | In combination with bortezomib (BTZ) and dexamethasone (DEX) for the treatment of patients with multiple myeloma (MM) who have received at least two prior regimens including BTZ and an immunomodulatory agent. | February 23, 2015 | March 24, 2022 |
Zydelig (idelalisib) | For the treatment of patients with relapsed follicular B-cell non-Hodgkin's lymphoma (FL) and small lymphocytic lymphoma (SLL) who have received at least two prior systemic treatment | July 23, 2014 | February 18, 2022 |
Imbruvica (ibrutinib) | For the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy | November 13, 2013 | May 18, 2023 |
Marqibo (vincristine sulfate liposomal) | For the treatment of adult patients with Philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia (ALL) that is in second or greater relapse or has progressed following two or more lines of anti-leukemia therapy | August 8, 2012 | May 2, 2022 |
Istodax (romidepsin) | For the treatment of patients with peripheral T-cell lymphoma (PTCL) who have received at least one prior therapy | June 16, 2011 | July 30, 2021 |
Oforta (fludarabine phosphate) | For adult patients with B-cell chronic lymphocytic leukemia (CLL) who have not responded to or have progressed during or after treatment with at least one standard alkylating-agent-containing regimen | December 18, 2008 | December 31, 2011 |
Avastin (bevacizumab) | In combination with paclitaxel for the treatment of patients with metastatic HER2-negative breast cancer who have not received prior chemotherapy for metastatic disease | February 22, 2008 | November 18, 2011 |
Bexxar (tositumomab and iodine i 131 tositumomab) | For patients with relapsed or refractory, low-grade or transformed CD20+ non-Hodgkin lymphoma (NHL) who have not received prior rituximab therapy | December 22, 2004 | October 23, 2013 |
Iressa (gefitinib) | As a single agent for the treatment of patients with locally advanced or metastatic non-small cell lung carcinoma (NSCLC) who have failed both platinum-based and docetaxel chemotherapies | May 5, 2003 | April 25, 2012 |
Mylotarg (gemtuzumab ozogamicin) | For patients 60 years of age and older with CD33+ acute myeloid leukemia (AML) who are in first relapse and not considered candidates for cytotoxic chemotherapy | May 17, 2000 | November 28, 2011 |
Celebrex (celecoxib) | As an adjunct to usual care for reducing the number of adenomatous colorectal polyps in patients with familial adenomatous polyposis | December 23, 1999 | June 8, 2012 |
From the tragic stories of these drugs, it can be seen that the U.S. FDA's procedures for accelerated approval and withdrawal of drugs are becoming increasingly refined. Both the pathways for approval of outstanding drugs and the exit routes for inferior drugs are very clear. In this regard, China's NMPA should take note and quickly perfect its own relevant withdrawal mechanisms.
1-FDA官网信息。
1-FDA official website.
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


