Shruti TalashiJanuary 02, 2024
Technological advancement apart from a deeper comprehension of disease biology, and an emphasis on breaking down conventional boundaries is driving this era of profound change in the drug development industry.
Traditional drug design methods have been successful in identifying drugs that currently treat Human immunodeficiency viruses (HIV) and cancer. With each drug taking an average of ten years to develop and 90% of drug candidates failing expensive clinical trials that can cost anywhere from $1 billion to over $2 billion per drug, researchers are searching for quicker and more effective ways to sort through possible drug molecules. With respect to this year, latest breakthroughs in AI-driven drug discoveries are discussed below.[1]
Artificial Intelligence (AI) is gaining traction in the field of drug discovery, and 2023 is the turning point for this technology. Using zero-shot generative AI in antibody creation, Absci Corporation announced at the beginning of this year that it has found three AI-created binders with a tighter binding affinity than the therapeutic antibody trastuzumab.[2] The release of MIT's DiffDock a diffusion generative model, which has a 38% success rate-higher than the conventional docking prediction techniques used in computer aided drug discovery (CADD) -could enable quicker, safer drug development. Although still in its infancy, this technique has demonstrated encouraging results in speeding up drug development and predicting the binding of proteins to ligands. By using this method, researchers at the University of Washington St. Louis are able to provide important insights into the potential efficacy of a novel drug candidate by characterizing its binding mechanism for aging-related disorders.[3]
A cloud service for generative AI-based drug development is called Nvidia's BioNeMo Cloud. Using proprietary data, researchers can refine models and carry out inference through the web or APIs. Customers of BioNeMo include AstraZeneca and a number of startups, such as Insilico Medicine and Evozyne. Evozyne declared that it had developed "supernatural" proteins using the service; that is, therapeutic proteins that may be more effective than naturally occurring ones for the rare metabolic disease phenylketonuria.[1]
Using its generative AI tool, InClinico, Insilico Medicine reported in August 2023 a substantial breakthrough in clinical trial outcome prediction. The algorithm was taught by the company using data from over 55,600 phase 2 clinical trials. Constructed over a span of seven years, InClinico's precision rate in predicting the results of actual phase 2 and 3 trials may establish the foundation for enhancing the efficiency of drug development with 35% return on investment in a virtual trading portfolio over a nine-month period.[4]
A deep learning (DL)-based framework is the University of Central Florida's known as BindingSite-AugmentedDTA model. Its goal is to improve drug-target affinity (DTA) predictions by finding possible protein binding sites as efficiently as possible. Through in vitro tests, the researchers have already confirmed the model's predictive ability. In order to anticipate the protein targets for 36 billion chemical compounds, Recursion is screening Enamine REAL Space, a searchable chemical library, utilizing Cyclica's MatchMaker technology, NVIDIA DGX Cloud supercomputing, and DeepMind's AlphaFold2 database. This method combines large-scale chemical databases and high-performance computing to assess if a tiny molecule will interact well with a protein binding site through machine learning. [1]
The first medication found by AI to enter phase 2 trials is INS018_055. Generative AI was employed by Insilico to find INS018_055, a tiny chemical that has just been included to a phase 2 research as a possible treatment for idiopathic pulmonary fibrosis. It's interesting to note that the business has also had success forecasting clinical trial results using generative AI.[5]
In two sizable phase 3 trials of acutely psychotic people with schizophrenia, "ulotaront" a trace amine-associated receptor (TAAR) agonist and potential schizophrenia medication developed by Sumitomo & Otsuka, did not perform better than a placebo, despite encouraging phase 2 results. Few publications in Nature make the case that data obstacles still stand in the way of fully utilizing AI in drug development. More and more people are starting to wonder if AI's promise for drug development and discovery is being exaggerated.[1]
Nevertheless, FDA has "accelerated its efforts to create an agile regulatory ecosystem" for AI that strives to balance innovation while protecting public health. The agency has addressed AI/ machine learning (ML) in drug development and in light of this it has published a number of guideline documents and discussion papers that address AI/ML in medication development and has not yet issued formal guidelines for the use of AI/ML in drug discovery. The FDA's approval of A2A Pharma's Investigational New Drug (IND) application for A2A-252, an inhibitor of TACC3 PPI in women's cancer, shows how small AI-enabled teams can move clinical stage products forward quickly.[6]
A huge step forward in the CRISPR research area of medicine was made on December 8, 2023, when the U.S. Food and therapy Administration (FDA) authorized the first gene-editing therapy ever. For the treatment of sickle cell disease (SCD), there is a novel medication known as Casgevy else known as exagamglogene autotemcel (exa-cel). Casgevy employs a cutting-edge gene-editing technique called CRISPR/Cas9, which enables researchers to precisely alter a patient's DNA.
A Phase 1/2 clinical trial is being conducted to assess the safety and efficacy of OTQ923, a similar SCD treatment being developed by Intellia Therapeutics and Novartis, in adults with severe SCD problems. There have been a few other updates regarding data releases, new trials, and biologics license application (BLA) submissions scheduled for 2023. For example, Editas medicine, known as EDIT-301, is being used to treat blood disorders like sickle cell disease (SCD). Additionally, beta-thalassemia is presently being studied in two Phase 1/2 clinical trials: the RUBY trial, which evaluates it in patients with severe SCD, and the EdiTHAL trial, which evaluates it in patients with transfusion-dependent beta thalassemia.
A Phase 1/2 clinical research is presently being conducted to assess the safety and effectiveness of Intellia Therapeutics' additional NTLA-2002 in patients suffering with hereditary angioedema (HAE), an uncommon and possibly fatal genetic condition that produces episodes of swelling in different regions of the body. Phase I clinical studies are presently being conducted for CRISPER Therapeutics' CTX310 therapy to treat dyslipidemia, a disorder marked by elevated triglyceride and cholesterol level.
Oncology gene editing is a rapidly expanding discipline, with many promising preclinical candidates positioned to transform cancer therapy. These medications have not yet entered clinical trials, yet they have truly revolutionary potential to prevent cancer, improve immunotherapy, and target specific mutations. The leading techniques for selectively infecting and killing cancer cells are created viruses based on the CRISPER technology, base editing for mismatch repair deficiency (MMR) cancers, and epigenetic editing for silenced tumor suppressor genes. This technology has the potential to revolutionize cancer treatment and we may anticipate even more cutting-edge gene-editing medications/ new modalities to make their way into clinical trials as research advances, bringing us one step closer to a day when cancer is no longer a death sentence.[6]
This year has witnessed a notable advancement in the creation of vaccines, providing hope for improved defense against both established and newly emerging infectious illnesses. The investigation of mRNA technology for various diseases, such as influenza, the Zika virus, and rabies, has been sparked by its use in COVID-19 vaccines. Since its introduction in late 2023, bivalent mRNA vaccines-which target both the original virus and Omicron variants-have been the recommended booster dose for the majority of people. Their protection against COVID-19 strains in circulation is more extensive.
The first-ever respiratory syncytial virus (RSV) vaccine for pregnant women may be approved by late 2023 or early 2024, marking a significant advancement. Newborns will be protected against this common respiratory infection by this immunization. There are several dengue vaccinations being developed, and several of them are progressing well in clinical testing. There is a vaccination that works against all four dengue serotypes in a single injection this will revolutionize the medical field. Although there isn't yet a perfect TB vaccine, there have been major developments in the field and continuous research is being done to enhance TB control and prevention. Developed a century ago, BCG (Bacillus Calmette-Guérin) remains the only TB vaccination that is currently licensed. Promising possibilities for the TB vaccine include MVA85A and rBCG. One method for developing TB vaccines is the subunit vaccine, which targets particular TB proteins rather than the entire live bacterium. They might provide wider protection and be safer than BCG. T-cell vaccines are an additional strategy that tries to activate the immune system's T cells, which are essential in the fight against tuberculosis. Antimicrobial resistance (AMR) is a worldwide danger. Although there is currently no vaccine specifically designed to treat AMR, fascinating research on a global scale is investigating the possibility of vaccinations to counteract the growing menace of antibiotic-resistant illnesses. Research on vaccines that target virulence factors the qualities that make bacteria harmful is being conducted using Next-generation vaccines. This could potentially lessen the severity of illnesses and the requirement for prolonged antibiotic treatment. Additionally, the goal of immune-stimulating chemicals combined with weakened bacteria in antibiotic-adjuvant vaccinations is to increase the immune response and maybe lessen the need for antibiotics. The development of an AMR vaccine is still in its infancy; to guarantee safety and effectiveness, extensive research and clinical trials are needed. There are difficulties in developing AMR vaccines since different bacteria acquire resistance in different ways, making it difficult to target specific resistance mechanisms. This makes it difficult to create a single vaccination that is effective against all AMR strains. In order to address antimicrobial resistance (AMR), worldwide cooperation between researchers, governments, and pharmaceutical corporations will be necessary. This cooperation will speed up the creation, distribution, and accessibility of effective vaccinations.[7]
There isn't a readily accessible, authorized vaccination to treat any human protozoan illness. Even though there are a lot of research and development activities going on, a number of obstacles have prevented the creation of effective protozoan vaccines. These include the complicated life cycle of protozoan infections, various strategies for evading the immune system, and a lack of funding for studies. Many countries are rolling out the first malaria vaccine approved by the WHO as vaccine RTS, S. R21 malaria vaccine doses are expected to reach countries in 2024. [8]
Developments in cell-based therapy saw a spike in 2023, offering promise for diseases that had no treatment before. CAR-T cell therapy evolved in oncology with efforts to treat solid tumors and bi-specific targeting. With the advent of gene editing capabilities and encouraging stem cell studies, neurodegenerative illnesses gained popularity. Point-of-care technology and engineered tissues added fuel to the fire, opening the door for a future in which cell-based therapies transform medicine and become widely available and beneficial for everyone. Future developments in technology, accessibility, and research yield great potential for the widespread use of cell-based therapies in healthcare. The FDA has not yet approved any cell-based treatments expressly for neurodegenerative illnesses. Even though this field of study is showing promise, there are still many obstacles to overcome due to the complexity of these illnesses and the difficulties in creating and evaluating cell-based treatments. The use of stem cells in treatment of neurodegenerative illnesses offers promise. Early trials investigate improving brain health in Alzheimer's disease and restoring damaged neurons in Parkinson's disease. Though they are still in the early stages of development, immune cell treatments and gene editing present additional potential approaches. The secret to realizing their potential and enhancing patient outcomes is ongoing research. Nevertheless, FDA is moving quickly to approve novel cell therapies because they have the potential to revolutionize cancer treatment. Patients with lymphomas and multiple myeloma can find hope in examples such as Kymriah, Abemac, Carvykti, Yescarta, and breakthrough therapy. The rush to transform cancer treatment is underway, even as alternative medicines like Tecartus and Tecfin gain traction.[9]
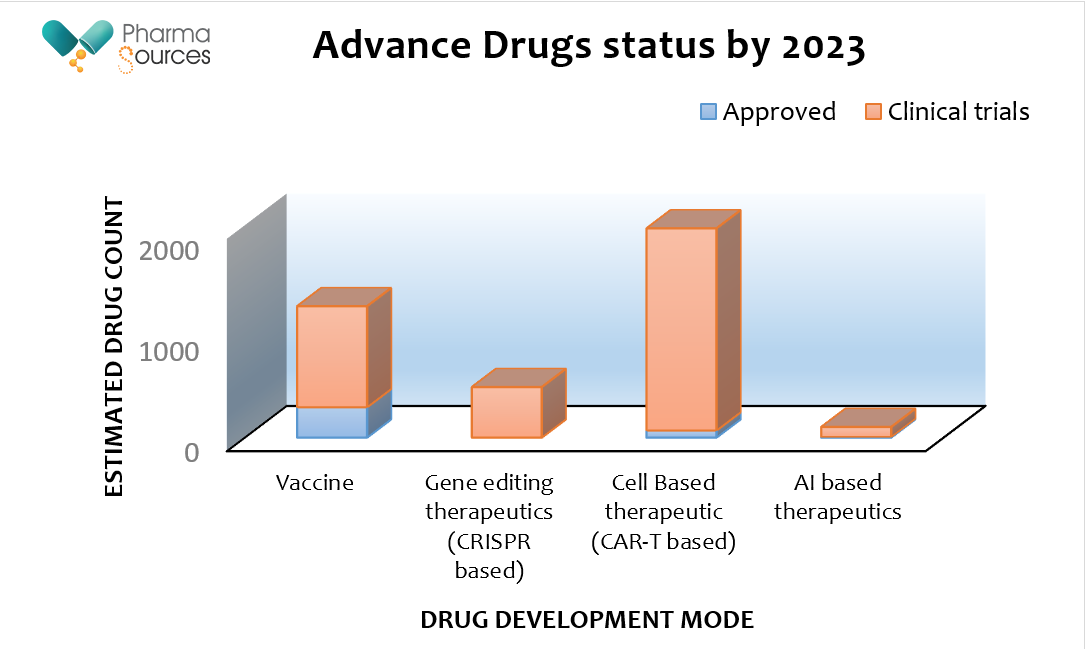
Figure represent estimated drug counts of approved vs. under clinical trials for the various drugs development modes while it is clearly seen that more biologics vaccines are available in market today and cell based therapies are dominant in the ongoing clinical trials 2023. Data has been gathered from & fda.gov & ClinicalTrials.gov websites.
Antibody-drug conjugates (ADCs) and Alphavirus-vectored virus-like particles (AVV/LVVs) are novel technologies with enormous potential in drug discovery and development, particularly in oncology. ADCs are making a big impact in cancer treatment in 2023, with new FDA approvals like as Enhertu and Jemperli indicating their potential. These innovative "smart bombs" combine antibody targeting with strong medicines, delivering a pinpoint hit to cancer cells but harming healthy tissues as little as possible. But the battle is far from over. Scientists are pushing the envelope with new ADCs that tout improved tumor selectivity, more potent payloads, and even the capacity to treat difficult solid tumors. Bispecific ADCs, which target multiple tumor antigens, have even more potential. ADCs are paving the way for a brighter future in cancer treatment.
While not yet household names, AVV/LVVs are quietly creating a reputation for themselves in the medical field. Unlike their infectious relatives, these designed viral particles transmit therapeutic genes or antigens directly to target cells, holding enormous promise for gene therapy, vaccinations, and even cancer-killing viruses. Though they are still in early trials, they have showed promising outcomes in a variety of areas, including neurodegenerative illnesses and infectious diseases. Researchers are working hard to improve their targeting, safety, and production in order to realize their full potential. Notably, AVV/LVV-based cancer vaccines are on the horizon, with the goal of conditioning our immune systems to become the ultimate tumor-fighting force. These small viral bundles could be the future of medicine, discreetly giving promise for a healthier tomorrow. ADCs & AVV/LVVs have production, economic, and side effect challenges, but their future appears promising as researchers perfect them and investigate their interaction with other technologies. The future of medicine lies in this collaborative approach, in which varied platforms such as ADCs and AVV/LVVs work together to develop tailored, effective therapeutics for a healthier tomorrow.[10]
Exciting new players like oncolytic viruses, therapeutic microbiomes, and proteolysis-targeting chimera (PROTACs) are emerging on the medical scene. Oncolytic viruses, like Amgen's Imlygic, offer targeted cancer cell destruction, while engineered microbiomes hold promise in tackling gut issues and beyond. PROTACs, with their ability to continuously degrade disease-causing proteins, are revolutionizing drug development, reaching targets previously deemed untouchable. These rising stars offer a glimpse into a future where diverse therapeutic modalities tackle a wider range of diseases, painting a brighter picture for healthcare.[10]
In conclusion, scientific advancements today is driven by exponential advancements in big data science, computational biology, artificial intelligence (AI), immunology, proteomics, metabolism, gut microbiomes, virology, and genomics. The emergence of CRISPR/Cas9 gene editing technology, ADCs, AVV/LVV has created a dizzying array of possibilities in customized and precision medicine. The availability of open access to the diverse and high-quality biological data online along with new AI/ML driven tools in drug design, drug lead and clinical trials are playing a crucial role in transforming and fastening the drug discovery and development.
1. Brian Buntz, Drug Discovery & Development 'A year in review: AI's evolving role in drug discovery and development in 2023'. Post date: October 23, 2023; Access date: December 12, 2023. URL: https://www.drugdiscoverytrends.com/ai-drug-discovery-2023-trends/
2. Absci, Absci Announces Collaboration with AstraZeneca to Advance AI-Driven Oncology Candidate. Post date: April 12, 2023; Access date: December 14, 2023 URL: https://investors.absci.com/news-releases/news-release-details/absci-announces-collaboration-astrazeneca-advance-ai-driven
3. Regina Barzilay & Tommi Jaakkola, Jameel Clinic 'DiffDock: Diffusion Steps, Twists, and Turns for Molecular Docking' Access date: December 14, 2023. URL : https://jclinic.mit.edu/research-project/diffdock-diffusion-steps-twists-and-turns-for-molecular-docking-2/
4. Ekambaram Gayathiri, Palanisamy Prakash, Priya Kumaravel et.al., Computational approaches for modeling and structural design of biological systems: A comprehensive review, Progress in Biophysics and Molecular Biology, 10.1016/j.pbiomolbio.2023.08.002, 185, (17-32), (2023).
5. Aliper A, Kudrin R, Polykovskiy D,et.al.,Prediction of Clinical Trials Outcomes Based on Target Choice and Clinical Trial Design with Multi-Modal Artificial Intelligence. Clin Pharmacol Ther. 2023 Nov;114(5):972-980. doi: 10.1002/cpt.3008. Epub 2023 Aug 10. PMID: 37483175.
6. A2A Pharmaceuticals Inc. ClinicalTrials.gov Identifier: NCT06136884 A First-In-Human, Phase 1 Study Evaluating Oral TACC3 PPI Inhibitor, AO-252, in Advanced Solid Tumors First update November 18, 2023; Last update November 18, 2023; Access date : December 14, 2023. URL : https://classic.clinicaltrials.gov/ct2/show/NCT06136884
7. Lindsay Smith Rogers, Game Changers: 5 Global Vaccine Innovations on the Horizon Published April 27, 2023; Access date December 14,2023. URL: https://publichealth.jhu.edu/2023/game-changing-vaccine-developments
8. World Health Organisation Malaria vaccines (RTS,S and R21) : What are next steps for roll out of the malaria vaccine and where will it be available? Post date: October 17,2023; Access date: December 14,2023. URL: https://www.who.int/news-room/questions-and-answers/item/q-a-on-rts-s-malaria-vaccine.
9. Bashor, C.J., Hilton, I.B., Bandukwala, H. et al. Engineering the next generation of cell-based therapeutics. Nat Rev Drug Discov 21, 655-675 (2022). https://doi.org/10.1038/s41573-022-00476-6
10. Mike Brochu, Lu Chen, and Brian Bush Biopharma / Article New Drug Modalities 2023 Post date: June 30, 2023; Access date: December 14,2023. URL: https://www.bcg.com/publications/2023/latest-industry-report-on-new-drug-modalities
Ms. Shruti Talashi boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


