Deepak HegdeDecember 27, 2023
Tag: Solubility , spray drying
ABSTRACT: This article, which is Part 2 of the series on address some of the practical considerations in application of spray drying to later phases of development, proprietary platforms in the industry, some products which have been commercialized for small molecules as well as application of spray drying to protein products, challenges and some products which have been commercialized for proteins.
The importance of using a QbD approach throughout an SDD product's life cycle, from development to validation, is critical owing to spray drying's multidimensional nature. The International Conference on Harmonization (ICH) defines QbD1 as "A systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management." A QbD approach to spray-drying process development starts with defining objective, which is the Target Product Profile (TPP) to ensure the safety and efficacy of the drug product. 1 The next step is to understand the relationships between ASD Critical Quality Attributes (CQAs) and Critical Process Parameters (CPPs) in the spray drying process so that a robust control strategy can be developed, drawing upon past product and process experience. Table 1 below lists the commonly identified CPPs for the spray drying process and their general impacts on CQAs 2,3,4,5,6,7.
Table 1: CPP's in spray drying and their impact on CQA's.
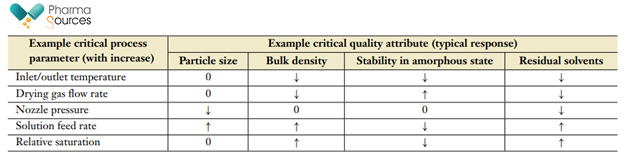
The examples shown in Table 1 may vary depending on the spray-drying process for each product however, understanding these interrelationships lays the groundwork for a successful control strategy. While applying QBD approach, the best approach is the development of an appropriate design space which can be used to define the multidimensional interaction of variables and parameters to demonstrate assurance of quality1
Development of an accurate design space for spray-drying process scale-up is vitally important. Maintaining physical stability of the amorphous form while producing materials that are amenable to robust manufacturing and performance of the dosage form process is critical.
Changes in manufacturing equipment at larger scale (e.g., nozzle size, chamber size, cyclone efficiency), shifts in thermodynamics (e.g., process temperatures), and differences in particle dynamics make fundamental understanding of these scale factors and functional product/process relationships key.
Spray-drying process and product functional relationships can be related through scale independent factors and/or fundamental models in key areas such as drying and thermodynamics or atomization and particle formulation 2,7
Several proprietary platforms have been used for both development as well as for commercialization purposes.8
The Hovione Intelligent Proprietary Screening methodology for ASDs (ASD-HIPROS®) was launched in January 2021 and is offered from the company's Lisbon, Portugal, R&D center (1). The service aims at developing ASDs formulations with maximum stability and performance by eliminating inviable candidates, thus saving the time and investment needed to bring the drug to the patient. Hovione claims that this proprietary process can assess up to 24 formulation prototypes in six weeks, requiring as little as five grams of API.
ZERION, a Danish drug development company established in 2019 as a spinout from the University of Copenhagen has pioneered the Dispersome® technology 9 that greatly enhances the solubility of poorly soluble, oral drugs and improves bioavailability and therapeutic outcomes for the patients. It is based on preparing stable amorphous formulations by mixing high loads of the drug compound with beta-lactoglobulin, a sustainable and biodegradable by-product from cheese production. ZERION develops proprietary drug formulations and offers the Dispersome® technology to established pharma companies to solve their most challenging drug solubility problems.
In China, Shenzhen Pharmacin Co. Ltd, a Shenzhen based company, established in 2016, has pioneered the use of SpraySolTM platform10. Pharmacin's SpraySol™ is an innovative and proprietary spray-drying, amorphous solid dispersion technology, which can enhance in vivo absorption of poorly soluble compounds that have high melting temperatures and high LogP values (highly hydrophobic). SpraySol™ can generate amorphous solid dispersions that are physically stable and create a favorable microenvironment for dissolution and maintaining supersaturation, thereby enhancing in vivo absorption. SpraySol™ technology provides greater improvement of in vivo absorption as compared to conventional amorphous solid dispersion technologies for small molecule compounds. This IP protected platform has been used for developing NCE's like PROTAC's as well as for developing 505b (2) products which have been demonstrated to reduce dosage compared to the innovator product, reduce/ eliminate food effects and reduce inter subject variations in clinical studies.
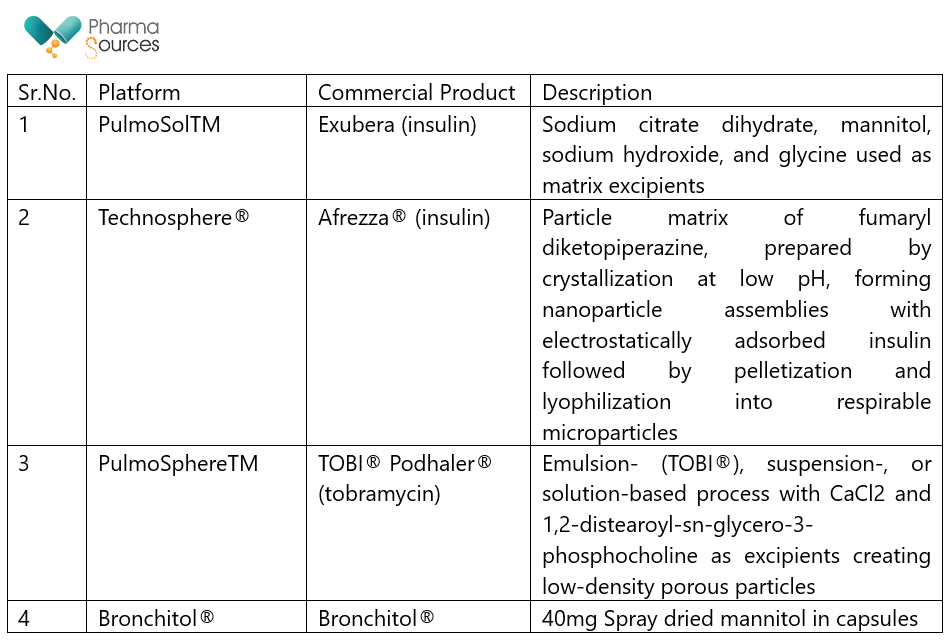
Some platform technologies developed for spray drying have also been successfully commercialized. Table 2 below describes current inhalation platform technologies developed for spray drying 11,12, 13,14
Table 2: Spray-dried platform technologies for inhalation
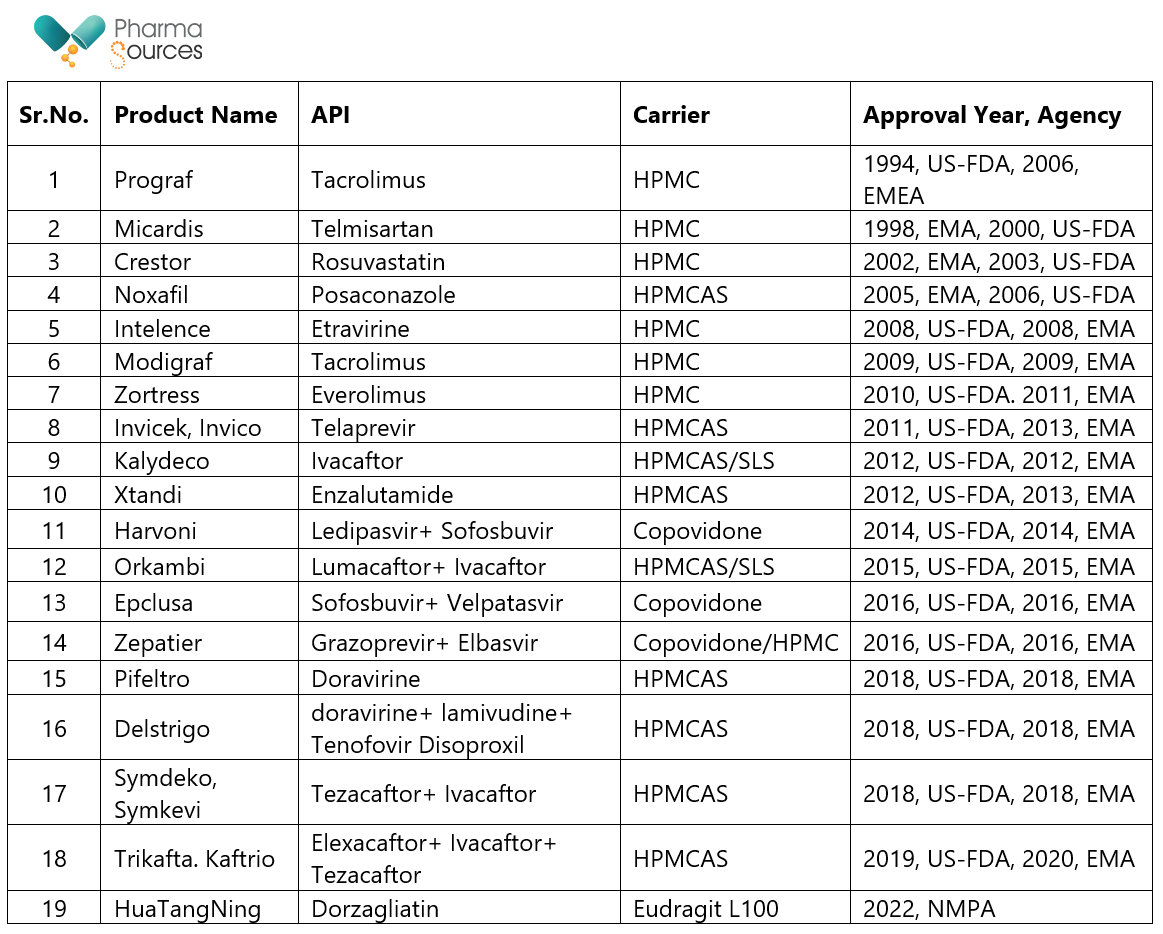
Spray drying has been successfully used in commercialization of small molecules. Table 3 below list below shows some of the products available on the commercial market, along with their approval dates and approving regulatory agency.15
Table 3: Commercial products of small molecules on market using spray drying technology
Many approved freeze-dried products are protein formulations (i.e. vaccines, antibodies, enzymes, peptide hormones, etc.) that emerged rapidly over the last years. Likewise, the expansion of alternative drying processes able to sustainably support the rapid development of biopharmaceutical technologies, are a central need of this industry in years to come. Unlike spray drying other technologies tend to be costly, time consuming, and not mature enough for industrial implementation. One of the main advantages of spray-drying, and a critical driver in generating the interest of the industry in this technique, is the possibility for "continuous processing." This provides a promising and rapid solution in terms of large volume manufacturing in particular cases like the recent crisis caused by the coronavirus disease 2019 (COVID-19).
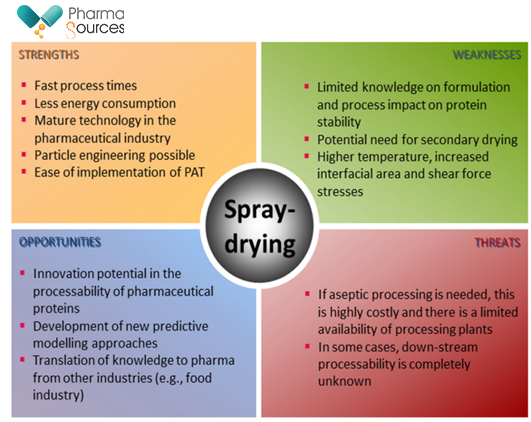
The figure below shows a generic analysis representing the strengths, weaknesses, opportunities, and threats (SWOT) associated with the spray-drying of protein pharmaceuticals.
Figure 1: SWOT analysis associated with the spray-drying of protein pharmaceuticals16
(Source: DRYING TECHNOLOGY 2021, VOL. 39, NO. 11, 1415-1446).
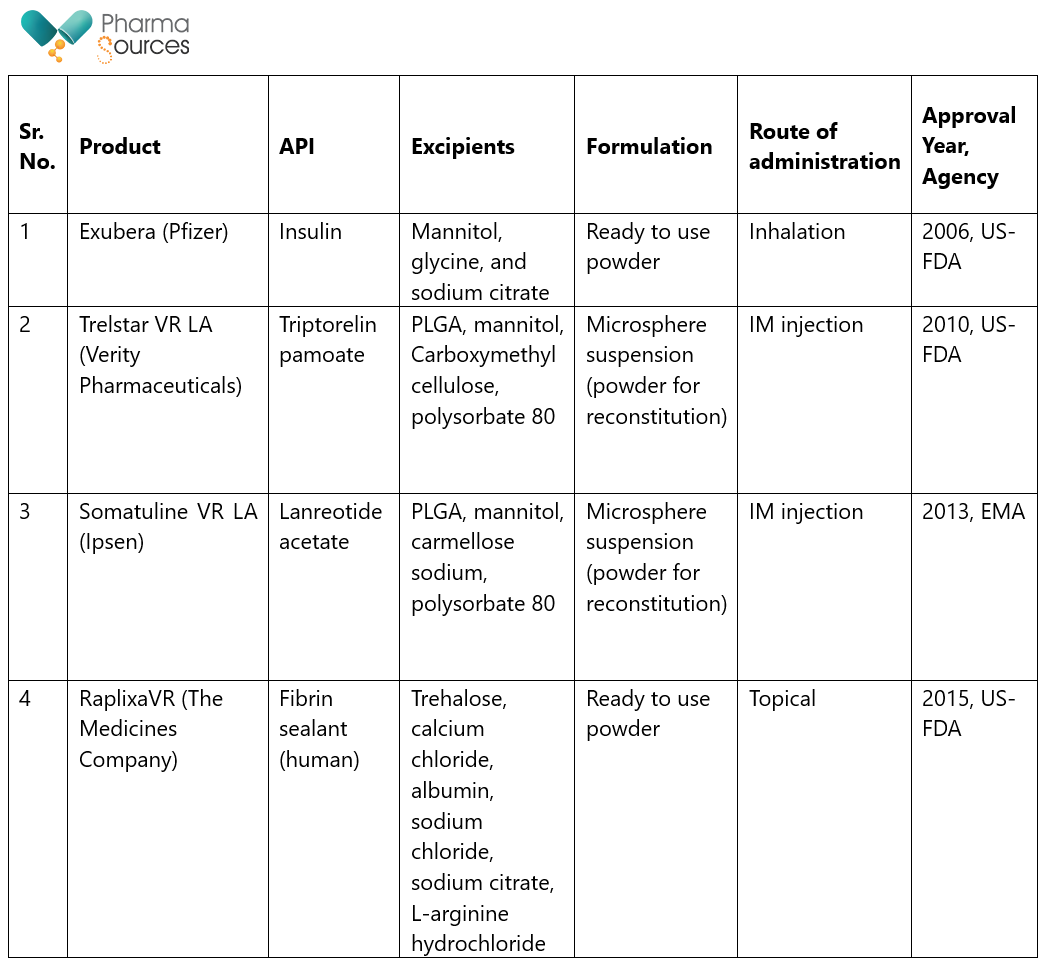
Spray drying has been successfully used in commercialization of proteins. Table 3 below list below shows some of the products available on the commercial market, along with their approval dates and approving regulatory agency.16
Table 4: Commercially approved protein pharmaceuticals produced via spray-drying.
(Source: DRYING TECHNOLOGY 2021, VOL. 39, NO. 11, 1415-1446).
Back to read:
1. US Food Drug Adm. Q8(R2) pharmaceutical development. Guide. Ind (2009).
2. Dobry DE, et al. J. Pharm. Innov. 4:133-42 (2009)
3. Gil M, et al. Chem. Today 28:18-22 (2010).
4. Ziaee Aet al. Eur. J. Pharm. Sci. 127:300-18 (2019)
5. Newman A, Pharmaceutical Amorphous Solid Dispersions, NJ: John Wiley & Sons (2015)
6. Poozesh S, et al. Int. J. Pharm. 562:271-92, (2019)
7. Lowinger Metal. Discovering and Developing Molecules with Optimal Drug-Like Properties, pp. 383-435. NY, Springer Verlag (2015)
8. Jennifer Markarian, Pharmaceutical Technology, Biopharma Outsourcing Innovation, Pages 22-29 (2022)
9. Zerion Pharma Press release, Feb 22, 2022.
10. Pharmacin Website, www.pharmacin.com/#our-technology
11. Al-Tabakha MM. 2015 J. Control. Release 215:25-38 (2015)
12. Geller DE, et al. 2011, J. Aerosol Med. Pulm. Drug Deliv. 24:175-82 (2011)
13. Kalia P. US FDA accepts Pharmaxis' Bronchitol application; November decision expected. S&P Global Market Intelligence, May 13 (2020)
14. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202049s000lbl.pdf
15. John Baumann et. Al. Annu. Rev. Chem. Biomol. Eng. 12:217-40 (2021)
16. Joana Pinto et al. Drying Technology, 39:11, 1415-1446

Deepak Hegde, Ph.D., M.F.M, is an industrial pharmacist by training. He has a been involved in development and commercialization of both innovative and generic drugs from a very early phase of development to technical transfers for commercial manufacturing sites, for the past 25 years. During his career, he has worked at Rhone Poulenc, Novartis (Sandoz), USV Ltd., WuXi AppTec, GSK & EOC Pharma. He is currently working with Shenzhen Pharmacin Co. Ltd. as Senior Vice President-Technology & Manufacturing.
Email: deepak.hegde@hlkpharma.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


