Shruti TalashiNovember 17, 2023
Tag: Dermatology drugs , healthcare sector , Skin cancer
The skin is a complex and important organ that plays a vital role in protecting the body from the environment and maintaining overall health. The US National Institute of Health reports that more than 3000 skin conditions are known to exist. Among these, the most prevalent and long-lasting conditions are skin cancer, psoriasis, acne, atopic dermatitis (AD), and allergic reactions like urticaria. These inflammatory skin diseases are common and can be long-lasting. They are caused by the activation of immune cells in the skin, which release cytokines that recruit other immune cells to the site of inflammation. Acute inflammation is a natural healing process, but chronic inflammation can be harmful.
Due to advancements in drug discovery research, a variety of inflammatory diseases are commonly treated with drugs for chronic inflammation, including Etanercept (Enbrel) a dimeric protein-based and Adalibumab (Humira) a monoclonal antibody-based, self-injectable drugs. It has been discovered that Infliximab (Remicade), an intravenous infusion medication based on monoclonal antibodies, works well for patients who do not respond to previous biologic therapy. Tumor necrosis factor-alpha (TNF-alpha), a protein involved in inflammation, is inhibited by these medications. Serious adverse effects like cancer, allergic reactions, and infections can also be caused by them. TNF-α is a pro-inflammatory cytokine involved in the chronic inflammation and can significantly impact on the metabolism and function of adipose tissue. Both Ixekizumab (Taltz) and Secukinumab (Cosentyx) are biologic medications constructed from monoclonal antibodies that are injected subcutaneously to treat moderate-to-severe plaque psoriasis and psoriatic arthritis. Both of these are monoclonal antibodies targets the cytokine interleukin-17 (IL-17), which is involved in inflammation. Ixekizumab was found to be more successful than Secukinumab in treating psoriatic arthritis in clinical trial. Both medications are typically safe and well-tolerated. Serious side effects, including severe infections, allergic reactions, and liver issues, can occur with severe case treatments. Other monoclonal antibodies based biologic medications such as risankizumab (Skyrizi) , guselkumab (Tremfya) , and tildrakizumab (Ilumya) share similarities with previous discussed biologic medicine in their formulation, use in disease treatment and adverse effects; nonetheless, they all target interleukin-23 (IL-23), a cytokine involved in inflammation. Risankizumab has a somewhat greater rate of total psoriasis lesion eradication. Cytoplasmic tyrosine kinases are known as Janus kinases (JAKs), it connect signal transducers and activators of transcription (STAT) transcription factors to cytokine signaling from membrane receptors. JAK inhibitors, which include the oral medications Upadacitinib (Rinvoq) and Ruxolitinib (Jakafi), function by preventing the action of JAK proteins that are implicated in the inflammatory response. These are safe and typically well-tolerated therapies for psoriasis and psoriatic arthritis. They may result in severe adverse effects like liver issues, blood clots, and dangerous infections. Above discussed injectable biologics drugs are used to treat moderate to severe Psoriasis.
On contrary, Topical corticosteroids (binds to glucocorticoid receptors GRs), Vitamin D analogues (binds to vitamin D receptors VDRs) & Calcineurin inhibitors (binds to calcineurin) are the class of medicine applied directly to the skin in the form of cream and it is known to effectively treat mild to moderate psoriasis. These medicines binds to their specific target protein and down-regulate the gene expression of the target protein hence lead to suppression of the immune system. There are drugs available for the treatment of most common skin inflammation caused by allergic reactions. A human monoclonal antibody called dupilumab which is administered by subcutaneous injection, targets the interleukin-4 receptor's alpha subunit (IL-4Rα). Numerous inflammatory and allergy disorders are influenced by the two significant cytokines, IL-4 and IL-13, whose signaling is mediated by this receptor. It is highly successful medication for moderate to severe AD also it is used to treat asthma. Although dupilumab is usually well tolerated, adverse effects such headaches, conjunctivitis, and injection site reactions are possible. Omalizumab is a recombinant humanized monoclonal antibody that targets immunoglobulin E (IgE), a protein implicated in allergic reactions. It is injected subcutaneously. In order to inhibit mast cells and basophils-cells that release inflammatory mediators upon coming into contact with IgE-from binding to IgE, omalizumab binds to IgE. This aids in lessening allergic reaction symptoms e.g. Utricaria. Omalizumab injection site responses, including redness, swelling, and itching, are the most frequent side effects. There have also been reports of headache, exhaustion, and nausea as side effects. An investigational medication called nemolizumab is used to relieve atopic dermatitis patients' itching. The interleukin-31 receptor A (IL-31Rα) is blocked by this monoclonal antibody. In reaction to allergens, mast cells and other immune cells release the cytokine IL-31. Nemolizumab is not yet licensed for use in humans and is currently in the clinical trial phase 2 stage of development. On the other hand, it might be a novel and useful treatment for atopic dermatitis sufferers' irritation.[1]
Skin cancer is the most common type of cancer in the world. Exposure to UV radiation from the sun is the primary risk factor for skin cancer. The World Health Organization has acknowledged that using sunscreen with an SPF of 30 can lower the risk of skin cancer in the general population. Skin cancer disease is categories broadly into two types (a) melanoma which is less common & yet aggressive type found in squamous cell, it develops anywhere in the body & (b) non-melanoma skin cancer (NMSC) most common & grows slowly, found in basal cell & squamous cells and can be cured with surgery. Now a days, skin cancer cases are becoming more common in many developing countries across the world. Imiquimod (Aldara) is a topical drug used to treat actinic keratosis and basal cell carcinoma. It attaches itself to immune cell toll-like receptors (TLRs), namely on dendritic cells and macrophages. This stimulates the immune system and causes the release of cytokines, including interferon-alpha (IFN-a ), which has an antitumor impact. A humanized antibody called pembrolizumab (Keytruda) is intravenous infusion drug used in cancer immunotherapy to treat melanoma, a kind of skin cancer that starts in the melanocytes. Pembrolizumab functions by obstructing T cells' surface PD-1 (programmed cell death-1) receptor. A protein called PD-1 aids in immune system regulation and keeps the body from attacking healthy cells. This drug increases the effectiveness of T cells' attack on cancer cells by inhibiting PD-1. Nivolumab (Opdivo) is another PD-1 inhibitor drugs available for the treatment of melanoma skin cancer or squamous cell carcinoma. Most of the skin diseases including skin cancer have inflammation in common hence there are various signaling pathways that are involved many production of cytokines. Cytokines inhibitors are believed to be essential in developing new drugs against broad range of skin diseases e.g. melanoma.[2,3]
Preclinical studies have demonstrated the efficacy of two IL-1RAs in preventing the growth and progression of melanoma: anakinra (Kineret) and canakinumab (Ilaris). Preclinical investigations have demonstrated that rilonacept (Arcalyst), an IL-1 Trap, effectively inhibits the growth and progression of basal cell carcinoma by binding to both IL-1α and IL-1β and preventing them from attaching to their respective receptors. For the treatment of skin cancer, IL-1 inhibitors are also being researched in conjunction with other treatments including immunotherapy.For new interventions in skin cancer, it crucial to investigate potential other cytokines e.g. ACKRs that are involved in the suppression of inflammation & regulation of multiple cancer cell signaling pathways. This is seen as potential therapeutic target as a promising drug and still being studied at a basic research level.[4]
The market for dermatology pharmaceuticals was valued at USD 17.24 billion in 2022 and is expected to reach approximately USD 51.32 billion by 2032, with a compound annual growth rate (CAGR) of 11.53% from 2023 to 2032. The psoriasis category led the market in 2022 with a revenue share of about 35.50%, and it is expected to grow at the highest CAGR of 11.8% from 2023 to 2032. The market for alopecia is projected to expand at the second-highest CAGR of 11.20% between 2023 and 2032. Alopecia is a disorder in which the immune system of the body targets the hair follicles, resulting in hair loss. Among the well-known companies in this market around the globe are Johnson & Johnson, AbbVie Inc., Novartis AG, Bausch Health Companies Inc., Bristol-Myers Squibb Company, Pfizer Inc., Galderma S.A., Eli Lilly and Company, Mylan N.V., GlaxoSmithKline plc, Sanofi, and LEO Pharma A/S.[5, 6]
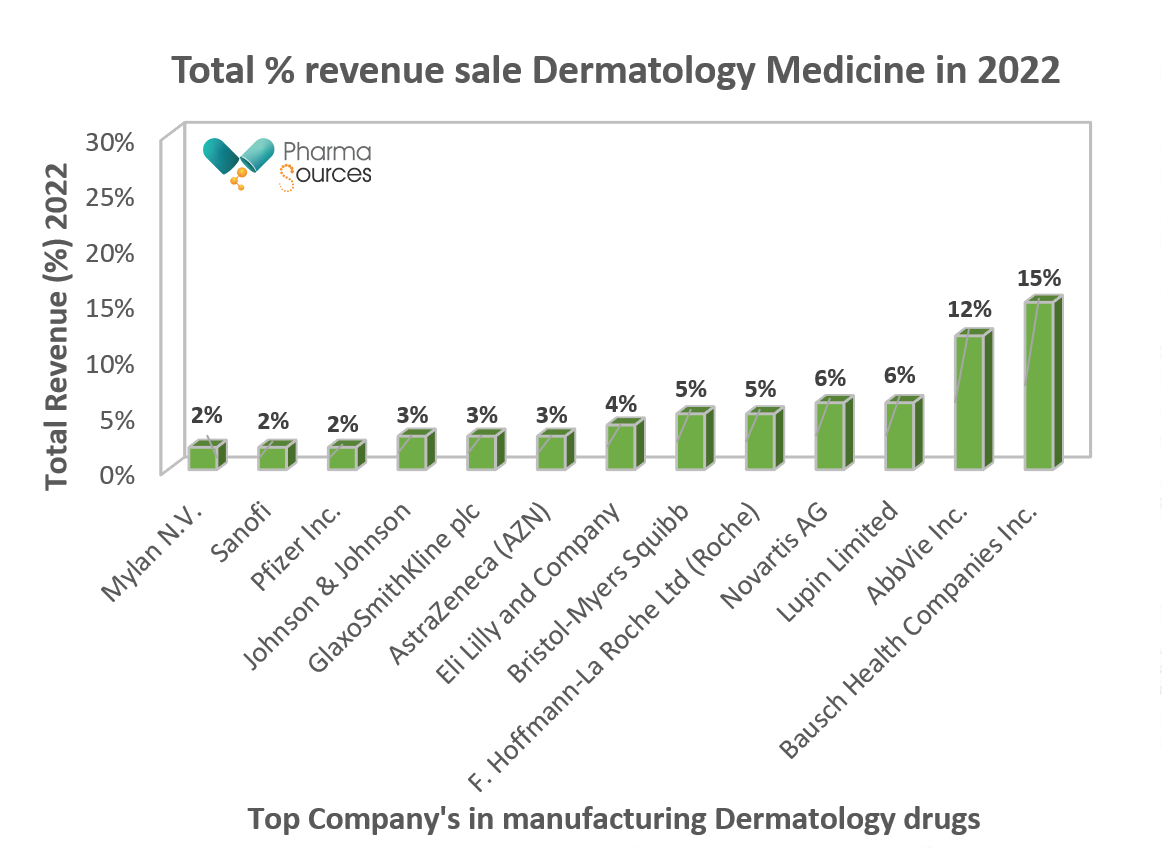
Figure 1. Above bar chart represent various companies total % sale revenue for dermatology medicine for the year 2022.
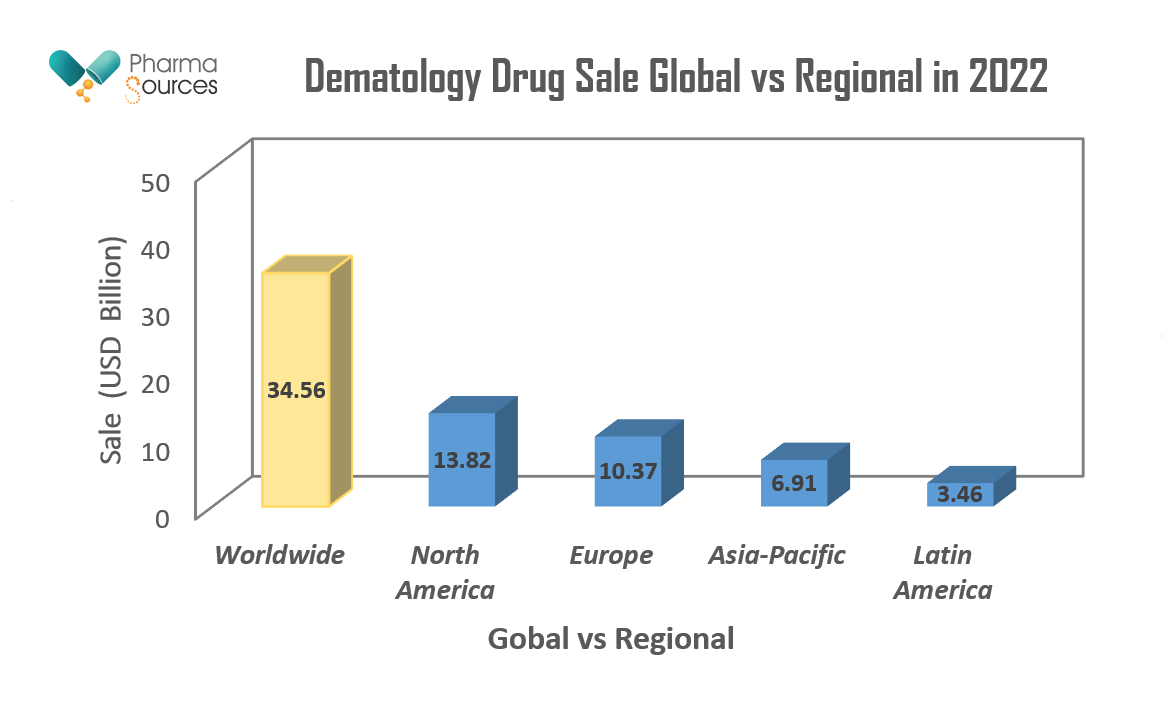
Figure 2. Above bar chart represent the total sale (USD) Billion of dermatology medicine worldwide and various regions of the globe for the year 2022.
Out of these Galderma S.A. & LEO Pharma A/S are the companies that solely focused on dermatology and has a wide portfolio of products for various skin conditions, including acne, rosacea, eczema, psoriasis, atopic dermatitis, and ichthyosis.
Tabe 1. Below table complies the top companies manufacturing various dermatology products and skin conditions.
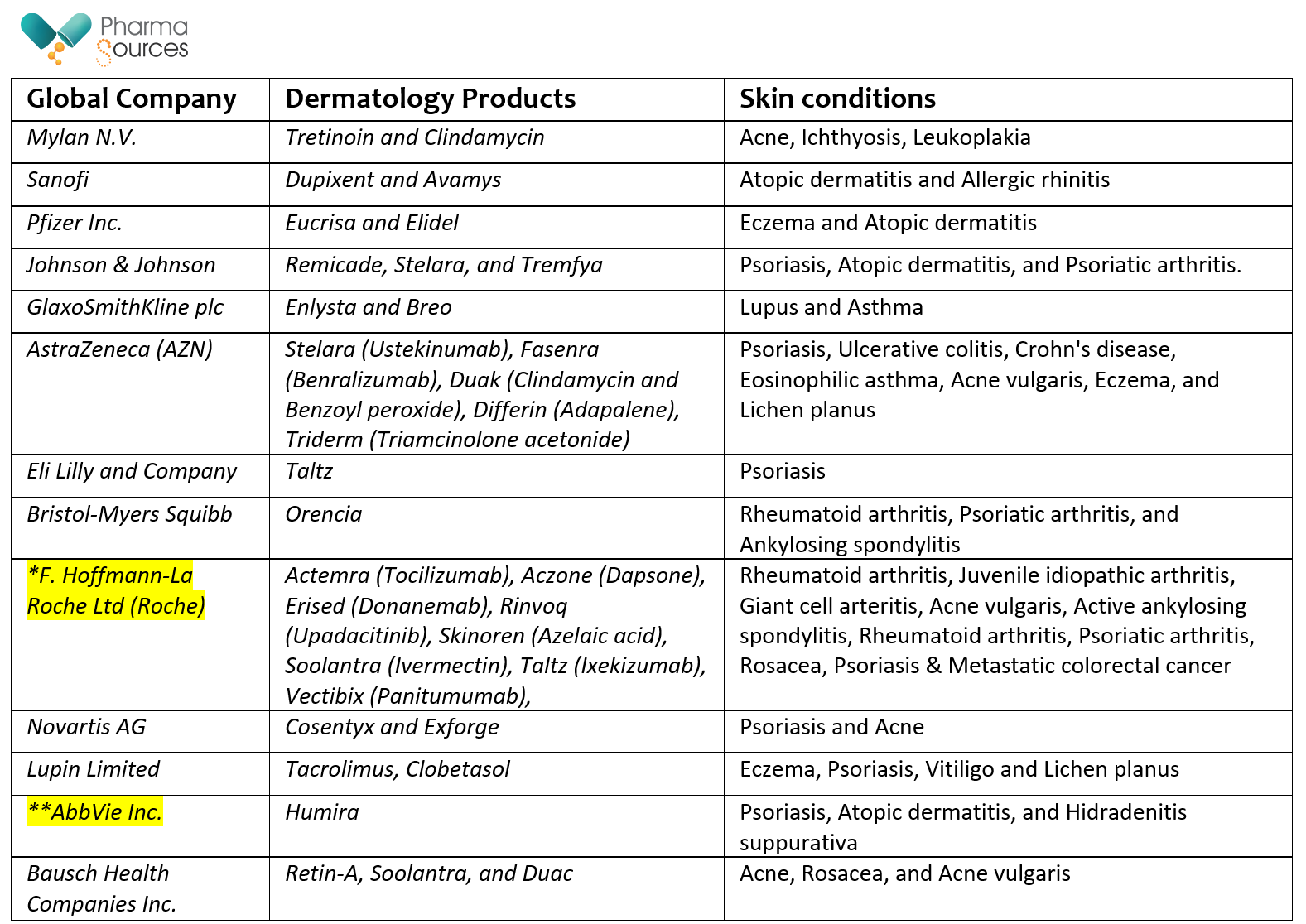
* company approved to sell dermatology related cancer drug ** company with highest sale revenue with single dermatology based product
Biopharmaceutical business Amgen Inc. creates and markets medications for a range of illnesses, such as bone disease, cancer, and anemia. Nevertheless, Amgen does not presently have any dermatology medications that are authorized for distribution in the US. Atopic dermatitis and psoriasis are two dermatology medications for which the company has been conducting clinical trials. However, the Food and Drug Administration (FDA) has not yet given these medications approval. Amgen purchased Evox Therapeutics in 2022; this business is working on a gene therapy for keratosis follicularis, a rare genetic skin condition. This would be Amgen's first dermatology medication to be sold in the US if the FDA approved it.[7]
All things considered, it is evident that in current market skin cancer related dermatology medicine are yet to be marked while it is useful to accelerate basic research and preclinical studies on the potential therapeutics in treating skin cancer as new intervention.
1. Aslam A, Griffiths CE. Drug therapies in dermatology. Clin Med (Lond). 2014 Feb;14(1):47-53. doi: 10.7861/clinmedicine.14-1-47. PMID: 24532745; PMCID: PMC5873621.
2. Mead MN. Benefits of sunlight: a bright spot for human health. Environ Health Perspect. 2008 Apr;116(4):A160-7. doi: 10.1289/ehp.116-a160. Erratum in: Environ Health Perspect. 2008 May;116(5):A197. PMID: 18414615; PMCID: PMC2290997.
3. Bridge JA, Lee JC, Daud A, Wells JW and Bluestone JA (2018) Cytokines, Chemokines, and Other Biomarkers of Response for Checkpoint Inhibitor Therapy in Skin Cancer. Front. Med. 5:351. doi: 10.3389/fmed.2018.00351.
4. Pacheco MO, Rocha FA, Aloia TPA, Marti LC. Evaluation of Atypical Chemokine Receptor Expression in T Cell Subsets. Cells. 2022 Dec 16;11(24):4099. doi: 10.3390/cells11244099. PMID: 36552863; PMCID: PMC9776531.
5. Global Dermatology Drugs Market Analysis. Insights 10 insight not just research, Last updated on: 21 June 2023, Accessed on 9 November 2023.
URL: https://www.insights10.com/report/global-dermatology-drugs-market-analysis/
6. Dermatology Drugs Market (By Indication: Acne, Psoriasis, Rosacea, Alopecia, Others; By Distribution Channel: Hospital Pharmacies, Retail Pharmacies, Online Pharmacies; By Administration Analysis: Topical Administration, Oral Administration, Parenteral Administration) - Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023-2032. Precedence Research, accessed on 9 November 2023.
URL: https://www.precedenceresearch.com/dermatology-drugs-market
7. Amgen Highlights Data to Be Presented at American Society of Clinical Oncology Annual Meeting Press Release May 15, 2013, accessed on 9 November 2023.
URL: https://www.amgen.com/newsroom/press-releases/2013/05/amgen-highlights-data-to-be-presented-at-american-society-of-clinical-oncology-annual-meeting
Ms. Shruti Talashi boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


