Yefenghong/PharmaSourcesNovember 07, 2023
Tag: Bispecific Antibody , BsAbs , Financing
Bispecific antibodies (BsAbs), also known as "dual antibodies," are artificially engineered antibodies prepared through techniques such as cell fusion, recombinant DNA, and protein engineering. They can simultaneously or sequentially bind to two different antigens or two different epitopes of the same antigen. Compared to monoclonal antibodies (mAbs), BsAbs offer advantages such as dual-targeted signal blockade, prevention of drug resistance, and enhanced internalization.
Due to the unique advantages of BsAbs, there has been an increasing number of players entering the field of bispecific antibodies. According to publicly available data, the number of clinical trials related to BsAbs has been continuously increasing at a rate of 20.44% per year. Currently, there have been 13 approved bispecific antibodies globally. What new developments are there in the field of bispecific antibodies this year?
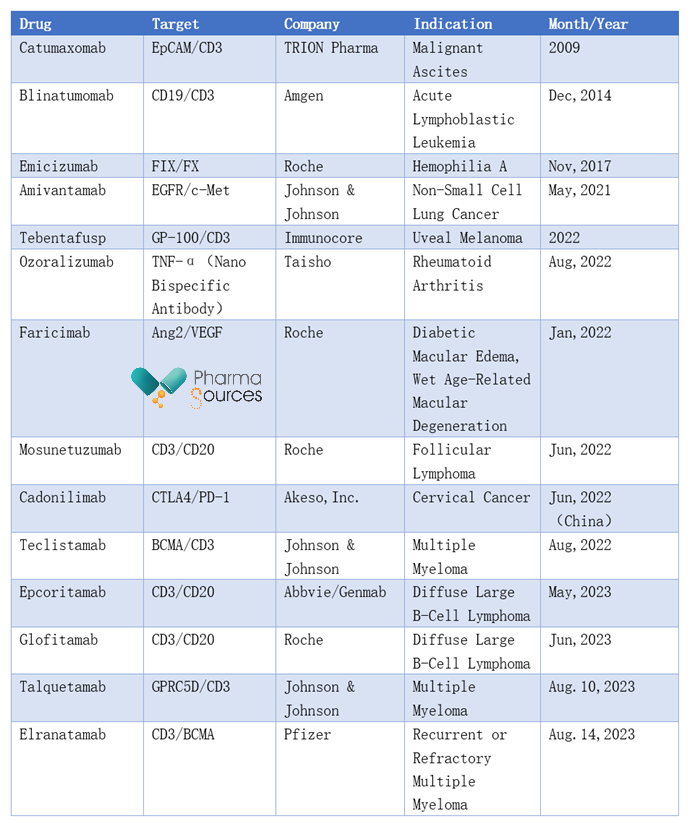
(Source: Publicly Available Information)
Since 2023, a total of four bispecific antibody drugs have been approved. On May 19, 2023, AbbVie and Genmab announced that the FDA approved their bispecific antibody drug Epcoritamab (trade name: Epkinly) for the treatment of relapsed or refractory diffuse large B-cell lymphoma. This drug is a CD3/CD20 bispecific antibody that can simultaneously target the CD20 protein on B cells and the CD3 protein on T cells to achieve the killing of CD20-positive cancer cells.
On June 15, 2023, the FDA approved Roche's Glofitamab (trade name: Columvi) for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or large B-cell lymphoma (LBCL) originating from follicular lymphoma in adult patients who have received at least second-line or higher systemic therapy. Epcoritamab is Roche's second CD3/CD20 bispecific antibody approved by the FDA.
On August 10, 2023, the FDA approved Johnson & Johnson's bispecific antibody drug Talquetamab (trade name: Talvey) for the treatment of relapsed or refractory multiple myeloma (MM) in adult patients who have previously received at least four treatments, including proteasome inhibitors, immunomodulatory agents, and CD38 antibodies.
Talquetamab is a GPRC5D/CD3 bispecific antibody. GPRC5D (G-protein coupled receptor family C group 5 member D) is an orphan receptor highly expressed on MM plasma cells. Its overexpression is closely related to poor prognosis and tumor burden in MM patients, and it is independently expressed from B-cell maturation antigen (BCMA). Talquetamab can activate T cells with CD3 receptors and induce their killing of GPRC5D-positive MM tumor cells, thus exerting an anti-tumor effect.
On August 14, 2023, the FDA approved Pfizer's Elranatamab (trade name: Elrexfio) for the treatment of relapsed or refractory MM patients who have previously received at least four therapies, including proteasome inhibitors (PI), immunomodulatory agents (IMiD), and anti-CD38 monoclonal antibodies.
Elranatamab is a humanized IgG2a T-cell-engaging bispecific antibody designed to target BCMA highly expressed on tumor cells and CD3 on T cells.
In addition to the four bispecific antibody drugs that have been approved and launched, the bispecific antibody field has also seen multiple financing activities this year. According to incomplete statistics, more than 20 Chinese companies in the bispecific antibody field have completed financing since 2023.
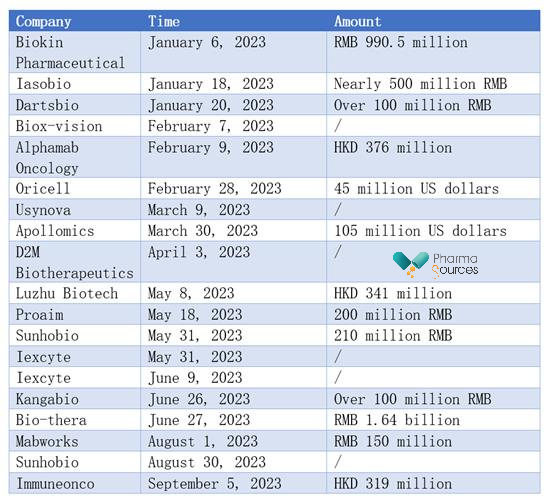
(Source: Publicly Available Information)
New progress has been made in the field of bispecific antibodies (BsAbs) in China, driven by capital investment.
Tebotelimab (MGD013), developed by Zailab, is a bispecific antibody that targets PD-1 and LAG-3, simultaneously blocking the PD-1 and LAG-3 signaling pathways to maintain or restore the function of exhausted T cells. It can be used to treat head and neck squamous cell carcinoma, gastric cancer, breast cancer, and diffuse large B-cell lymphoma, among others. In November 2018, Zailab Pharmaceuticals entered into a collaboration with MacroGenics to obtain exclusive development and commercialization rights for Tebotelimab in Greater China.
In a study conducted this year on patients with advanced hepatocellular carcinoma (aHCC) who had previously failed targeted therapy and/or immunotherapy, 13 patients received Tebotelimab treatment during the dose escalation phase, and no dose-limiting toxicities were observed. The objective response rate (ORR) in the study was 3.3%, and the disease control rate (DCR) was 50.0%. The median duration of response (DOR) in the immune checkpoint inhibitor (ICI)-treated group was 9.2 months, while only 4 patients in the ICI-naive group responded, with a median DOR of 5.8 months. The median progression-free survival (PFS) in the ICI-treated group and the ICI-naive group were 2.4 months and 3.1 months, respectively, and neither group reached the median overall survival (OS).
Elranatamab (KN046) from Alphamab is a humanized IgG2a bispecific antibody that targets BCMA highly expressed on tumor cells and CD3 on T cells. It is being developed for the treatment of relapsed or refractory multiple myeloma (MM). Elranatamab has been granted orphan drug designation by the FDA and EMA for the treatment of MM. Additionally, the FDA has granted it fast track and breakthrough therapy designations for relapsed or refractory MM, while the EMA has granted it PRIME (PRIority MEdicines) designation.
Iexcyte, a company that received two rounds of financing in the first half of this year, has developed a bispecific antibody called YK012, which targets CD3 and CD19. It is intended for the treatment of non-Hodgkin lymphoma and relapsed or refractory precursor B-cell acute lymphoblastic leukemia (ALL). YK012, based on the company's proprietary FIST platform, officially initiated phase I clinical trials in May of this year.
Luzhu Biotech based on its Fabite technology platform has developed bispecific products K193 and K333. K193 is a bispecific antibody targeting CD19/CD3 and is currently undergoing phase I clinical trials in China, with an expected completion in the fourth quarter of this year. The plan is to complete phase II clinical trials in the fourth quarter of 2027 and submit a New Drug Application (NDA) to the Center for Drug Evaluation (CDE) in 2027. K333 is a bispecific antibody targeting CD33/CD3 and is currently in preclinical studies.
Since the concept of bispecific antibodies was first proposed by Nisonoff and colleagues in 1960, BsAbs have become a dream pursued by scientists. With the development of monoclonal antibody technology, genetic engineering, and various bispecific antibody platforms, both domestically and internationally, the market for bispecific antibodies is rapidly expanding. In the future, the field of bispecific antibody research and development will witness more and more clinical breakthroughs.
1. U.S. Food and Administration. FDA grants accelerated approval to epcoritamab-bysp for relapsed or refractory diffuse large B-cell lymphoma and high-grade B-cell lymphoma. Accessed May 24, 2023.
2. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-talquetamab-tgvs-relapsed-or-refractory-multiple-myeloma.
3.https://investors.pfizer.com/Investors/News/news-details/2023/Pfizers-ELREXFIO-Receives-U.S.-FDA-Accelerated-Approval-for-Relapsed-or-Refractory-Multiple-Myeloma/default.aspx.
4.https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/761309s000lbl.pdf.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


