Shruti TalashiSeptember 19, 2023
Tag: ICH Q5C , ICH Q1 , Stability Testing
Stability testing is the process of analysing and monitoring the active pharmaceutical ingredients/APIs or the final pharmaceutical products/FPPs stability over time to ensure that it stays safe and effective during its shelf life and under that labelled storage conditions. The various guidelines including the stability testing for the new drug substance and products is provided in the ICH Q1 A-F and guidelines including the stability testing for the biotechnological/biological products is provided in ICH Q5C. A stability programme is a thorough plan for assessing and monitoring the long-term stability of a medicinal product that is under the post-marketing/ Phase IV of the clinical trials. Its purpose is to ensure that the medication product remains safe and effective throughout its proposed shelf life with storage condition. [1]
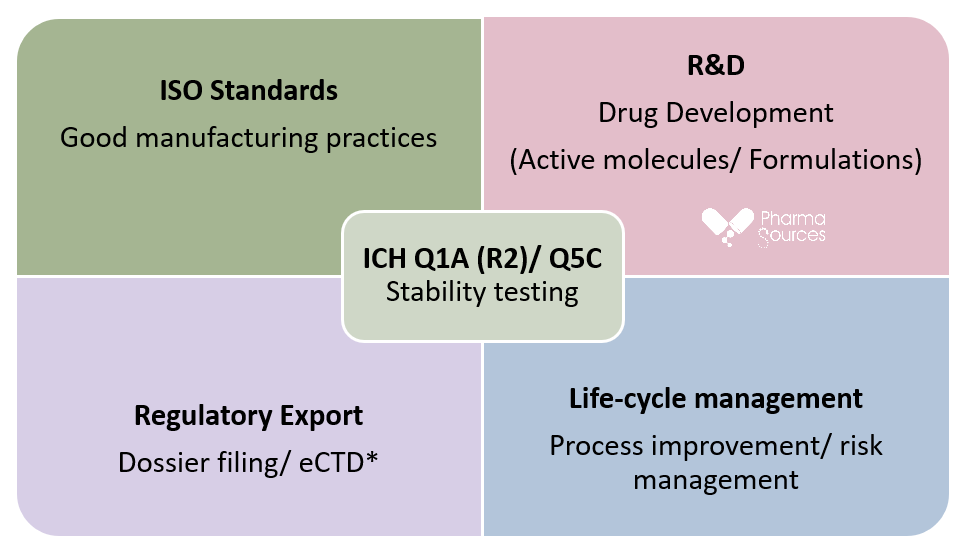
Figure above represents a matrix chart with stability testing shown in the centre as it plays a crucial part with supporting the pharmaceutical industry’s research and development R&D and manufacturing units in the drug development, process improvement and risk management, also it is vital for dossier filing and compliance with GMP. * eCTD full form is electronic common technical data, it consists of 5 modules out of which stability report is essential part in the module 2.
The stability of an API is frequently greater than that of the FPP. This is due to the fact that the API is often a pure and active product, but the FPP comprises additional substances such as excipients, added flavours as per the drug manufacturing formula DMF and packaging materials such as foils/containers. These additional substances may interact with the API, affecting its stability. Excipients, for example, can absorb moisture or oxygen, which can damage the API. Packaging materials can also interact with the API by leaching chemicals into the product, for example. Furthermore, the FPP is often subjected to greater environmental pressures than the API. During storage, shipping, and dispensing, for example, the FPP may be exposed to light, heat, and humidity. These pressures can potentially hasten the deterioration of the API. As a result of these elements, the FPP appears to have shorter shelf life than the API.
Stability testing as per the ICH Q1 A (R2) is applicable to new drug substance and products while ICH Q5C has the stability testing guidelines for the biotechnological/biological products. Stability studies are a critical component of the medication development and manufacturing processes. They help to ensure drug quality, safety, and efficacy, and they are necessary for drug export to other nations. Stability summary report or stability batch report is crucial document for dossier filing with the drug regulatory bodies hence it is very essential to work hard on the stability programme along with considering the methods for cost reduction since stability testing are stretched to a long term and frequent testing studies. [2]
The protocol for a drug substance's stability study is generally comparable to the protocol for a final pharmaceutical product's stability study. Yet there exists some differences like in the storage conditions and analytical methods. The analytical procedures used in drug substance stability studies are often more complex than those used in final pharmaceutical product stability studies. This is due to the fact that drug ingredients decay faster than finished pharmaceutical products, and the breakdown products may be more difficult to identify. Protocol for the FPP stability study includes:
Product details: The protocol should include the final pharmaceutical product's strength, formulation, and packaging.
Storage settings: The protocol should specify the storage conditions for the study, such as temperature, humidity, and light.
Sampling: The protocol should specify when and how samples will be collected for analysis.
Analytical procedures: The protocol should describe the analytical methods that will be used to test the stability of the finished pharmaceutical product.
Acceptance criteria: The protocol should specify the acceptance criteria that will be used to determine whether or not the finished pharmaceutical product is stable. This is crucial in delivery the COA, certificate of analysis of the stability sample.
In any well-defined testing programme intended to establish their stability for the desired storage duration, consideration should be paid to the distinctive properties of biotechnological/biological products related to ICH Q5C. The maintenance of molecular shape and, consequently, biological activity for such products, in which the active ingredients are typically proteins and/or polypeptides, depends on non-covalent as well as covalent forces. The environment's temperature variations, oxidation, light, ionic content, and shear are among the environmental elements that the products are particularly susceptible to. Stringent storage conditions are typically required to maintain biological activity and prevent degradation. The biotech companies filing dossiers for biologics/biosimilars should develop the proper supporting stability data for a biotechnological/biological product and take into account many external conditions that can affect the product's potency, purity, and quality.[2]
Since there exists an ambiguity in the ICH Q1 appendix/annexures and less details in ICH Q5C hence ICH Q1/Q5C will be reorganised into a core guideline with topic-specific annexes/appendices to explain which elements of the guideline apply to certain product types in the update. It will also help to increase harmonisation by removing apparent ambiguity. This will be define the analytical procedure and acceptance criteria for different types of products. [3]
The frequency of sampling or testing is critical in the stability protocol; the most common method is to have one short term accelerated temperature/humidity i.e. 40ºC ± 2 ºC/75% RH ±5% RH condition for study sample at 0, 3 & 6 month points and including three one year long term climate zone temperature/humidity condition i.e. 30ºC ± 2 ºC/75% RH ±5% RH or 30ºC ± 2 ºC/60% RH ±5% RH for study sample at 3 months intervals i.e. 0, 3, 6, 9, and 12 month points for at least three exhibit batch. The data from the stability batch report is essentially required in filing the dossier with the regulatory bodies prior to obtaining a clearance of drug export to other nations. This process of having multiple time points for three batches of each FPP’s as full study design in the stability programme has been recognised as a time & resource consuming processes.[4]
As per ICH 1QA (R2) unless bracketing or matrixing is used, stability studies should be conducted on each unique strength and container size of the drug product. Two reduced study design types that can be utilised for stability testing are bracketing and matrixing. A reduced research design is one in which not all samples are evaluated at each time point for each factor combination. When there are numerous design considerations at play, reduced study designs can be a good substitute for complete study designs. The risks of utilising a limited study design, such as the danger of establishing a shorter retest period or shelf life than may be obtained from a full study design, must be carefully considered. FPP’s having a lower risk of degradation or those with a shorter shelf life may be suited for reduced research designs like bracketing and matrixing. Any shortened study design should have a good reason for being used. The circumstances listed in the ICH Q1D guideline may, in some circumstances, be adequate reason for use. Bracketing and matrixing is explained with respect to the design factors and design consideration. In bracketing, stability study for drugs with multiple strengths of identical or closely related formulation is considered. In a bracketing design, samples are evaluated at all-time points for the most extreme values of each design component. Each design factor's remaining values are presumptively as stable as their bracketing values. Bracketing normally shouldn't be used when different excipients are employed between strengths. In a matrixing design, samples are evaluated at every time point for a portion of all potential factor combinations. It is presummated that the remaining factor combinations will be equally stable to the tested factor combinations. With justification, matrixing designs can be used, for instance, for various strengths where the proportions of the active ingredient and excipients fluctuate, or when several excipients are employed, or for various container closure systems. In general, justification should be supported by evidence. Supporting data could be provided, for instance, to compare the relative moisture vapour transmission rates or equivalent light protection between two distinct closures or container closing systems. Alternately, proof that the medication product is unaffected by air, moisture, or light could be provided. When identifying differences in rates of change across components and establishing a trustworthy shelf life, a study design that matrixes on time points alone is frequently just as effective as a full design. This is due to the fact that linearity is typically assumed in stability studies and that all factor combinations are still examined at the beginning and end of the study unlike in bracketing. [5]
There are challenges associated with adopting limited study designs in stability studies like lesser data collection, the correct justification for dossier filing, due to the complexity the risk of error while designing the sample frequency for the stability testing & less flexibility for correction from any deviation. Despite these challenges, limited study designs can be a valuable tool for stability testing, especially for products with a large number of design factors or for products with a short shelf life especially for biologics/bio similar products. In order to mitigate these challenges, a risk based approach will be useful in reduced design study. With the support of specialised biostatisticians and subject matter experts SME team a justification should be proposed based on only the scientific principle and supported by data.
1. ICH HARMONISED TRIPARTITE GUIDELINE STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS Q1A(R2) Current Step 4 version dated 6 February 2003. *Q1A(R2) Guideline.pdf (ich.org)
2. ICH HARMONISED TRIPARTITE GUIDELINE QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS Q5C Current Step 4 version dated 30 November 1995. *Q5C Guideline.pdf (ich.org)
3. Final Concept Paper Targeted Revisions of the ICH Stability Guideline Series (Guidelines ICH Q1A-F, ICH Q5C) Endorsed by the Management Committee on 15 November 2022. ICH_Q1Q5C_ConceptPaper_Final_2022_1114.pdf
4. ICH HARMONISED TRIPARTITE GUIDELINE STABILITY TESTING FOR NEW DOSAGE FORMS Annex to the ICH Harmonised Tripartite Guideline on Stability Testing for New Drugs and Products Q1C Current Step 4 version dated 6 November 1996. Q1C Guideline.pdf (ich.org)
ICH HARMONISED TRIPARTITE GUIDELINE BRACKETING AND MATRIXING DESIGNS FOR STABILITY TESTING OF NEW DRUG SUBSTANCES AND PRODUCTS Q1D Current Step 4 version dated 7 February 2002. *Q1D Guideline.pdf (ich.org)
Ms. Shruti Talashi boasts a dual mastery of lab research and writing. Her doctoral study outcome as M.Phil in biomedical science while studying breast cancer and an extraordinary masters degrees dissertation work on exploring role of Gal-lectin in cancer metastasis fuels her extensive research interests. She has gained few publication in journals. Bridging the science-public gap is her passion, aided by expertise in diverse techniques. From oncology to antibiotic/drugs production, she's led and managed complex projects, even clinical trials. Now, as a freelance Content Coordinator for Sinoexpo Pharmasource.com, her industry knowledge shines through valuable insights on cutting-edge topics like GMP, QbD, and biofoundry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


