Xiaoyaowan/PharmasourcesSeptember 08, 2023
Tag: hepatitis B , antisense oligonucleotide , functional cure
Recently, Haobo Pharmaceuticals announced that their investigational antisense oligonucleotide (ASO) drug AHB-137 has received clinical trial approval from the FDA in the United States for use in patients with chronic hepatitis B.
Antisense oligonucleotides (ASOs) are single-stranded molecules that, under the catalysis of RNase H1, bind to target mRNA based on the principle of base complementarity, resulting in mRNA degradation and ultimately inhibiting protein expression.
AHB-137 is a naked antisense oligonucleotide (ASO) and is also the first innovative drug from Haobo Pharmaceuticals' self-developed platform, Med-Oligo, to enter clinical trials, aiming to achieve functional cure for chronic hepatitis B. Haobo Pharmaceuticals announced its preclinical data at the 2023 Annual Meeting of the European Association for the Study of the Liver (EASL).
Currently, AHB-137 is undergoing clinical trials simultaneously in New Zealand and China. The FDA's clinical approval is part of a multi-regional, randomized, double-blind, placebo-controlled clinical trial to evaluate the safety, tolerability, pharmacokinetics, and preliminary efficacy of the drug in patients with chronic hepatitis B.
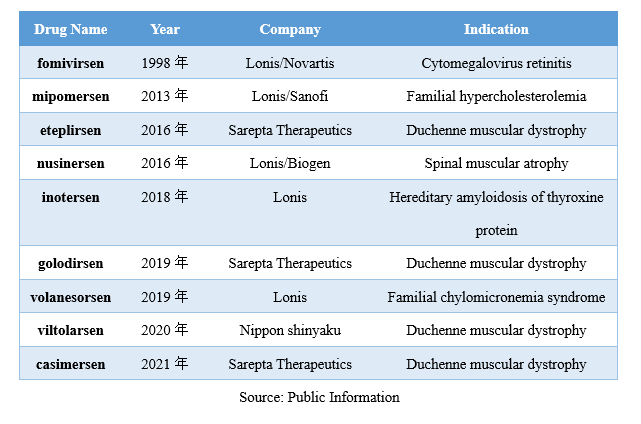
Globally, the first ASO drug, fomivirsen, was approved for the treatment of cytomegalovirus retinitis in 2018. Since then, eight ASO drugs have been successively launched in the global market, mainly targeting rare diseases. Among them, fomivirsen and mipomersen have been discontinued.
The best-selling ASO drug among those available is Nusinersen (trade name Spinraza), which is owned by Ionis/Biogen and is used for the treatment of spinal muscular atrophy, with annual sales reaching 2 billion US dollars.
In 2018, Nusinersen entered the first batch of urgently needed overseas new drugs list issued by the China National Medical Products Administration and subsequently obtained clinical exemption qualification. In 2019, Nusinersen was approved for marketing in China.
According to statistics, in 2022, the number of hepatitis B patients in China is about 18 million, among which the proportions of primary liver cancer and cirrhosis patients caused by HBV virus infection are as high as 80% and 60%, respectively.
The mainstream drugs for hepatitis B treatment include nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and interferons. After discontinuation of NRTI, patients will experience rebound, so hepatitis B patients need to take medication for life to suppress virus replication; at the same time, renal toxicity and bone toxicity associated with NRTI treatment are unavoidable. Ordinary interferon has a short half-life and requires frequent injections; there is also a problem of patient intolerance.
In summary, the above two types of mainstream drugs rarely achieve functional cure for hepatitis B, and patients basically need to take medication for life. There is an urgent clinical need for innovative therapies that can achieve functional cure for hepatitis B.
In the gene structure of HBV, the relaxed circular double-stranded DNA genome composed of some components can be transcribed into RNA of different lengths; among them, DR1 and DR2 are highly conserved two segments in the HBV genome. Existing research results show that small nucleic acid drugs specifically designed for these two segments can induce mRNA degradation.
The antisense oligonucleotide (ASO) drug AHB-137 mentioned at the beginning belongs to the category of the above innovative therapies. In addition to AHB-137, current clinical data have verified that small nucleic acid drugs are effective in reducing HBsAg, and the effect is better when used in combination therapy.
Bepirovirsen, an ASO drug jointly developed by GSK/Lonis, is currently in phase IIb clinical stage; another representative of innovative therapies is RNAi drugs, among which JNJ-3989 developed by Johnson & Johnson, VIR-2218 developed by Vir/Alnylam, AB-729 developed by Arbutus, and RO7445482 developed by Roche are in phase II clinical stage.
The current clinical results of these innovative therapies under development show that there are certain challenges for monotherapy to achieve functional cure for hepatitis B, and feasible treatments tend to be combination therapy. For example, in a phase II clinical trial, VIR used the small nucleic acid drug VIR-2218 in combination with neutralizing antibody VIR-3434, showing that the absolute value of HBsAg in most patients at the end of treatment did not exceed 10 IU/mL, and the efficacy of this result was significantly better than that of VIR-2218 monotherapy.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


