Dopine/PharmaSourcesAugust 30, 2023
Tag: Bispecific Antibodies , Target , PD-1/CTLA-4
According to the Pharmacodia Database, currently, there have been 13 drugs approved worldwide in this field. However, Removab was withdrawn from the market in 2017 due to its strong immunogenicity and commercial reasons.
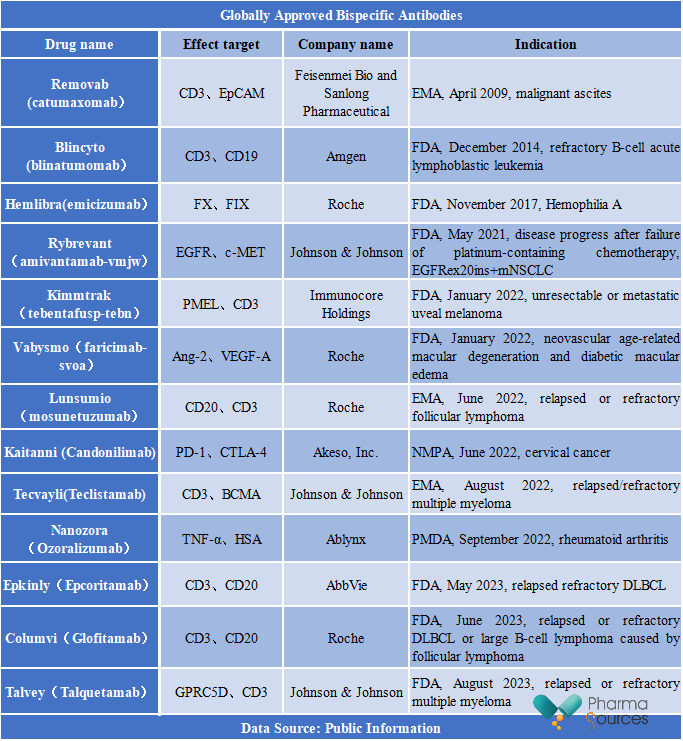
The effect targets of approved bispecific antibodies are relatively diverse, of which, 3 bispecific antibodies are approved for CD20/CD3 target. From a company perspective, foreign pharmaceutical companies, especially large transnational ones, are leading the field of bispecific antibodies. Among them, Roche has developed 4 bispecific antibodies, while Johnson & Johnson has 3 in the portfolio. In terms of indications, the approved indications of bispecific antibodies cover a wide range, involving hematologic tumors, solid tumors, ophthalmic diseases, autoimmune diseases, hemophilia and among others.
In addition, there are two bispecific antibodies currently under regulatory review for market approval globally. These include Elranatamab (PF-06863135) developed by Pfizer, which targets at BCMA/CD3 and is intended for the treatment of relapsed/refractory multiple myeloma. The other one is Ivonescimab (AK112/SMT112) developed by Akeso, Inc., which targets at PD-1/VEGF and is intended for the treatment of advanced nonsquamous NSCLC with EGFR mutations that has progressed after EGFR-TKI therapy.
Compared to other countries, China only approved three bispecific antibodies. These include Hemlibra (emicizumab) from Roche (approved in November 2018), Blincyto (blinatumomab) from Amgen (approved in December 2020), and Kaitanni (Candonilimab) from Akeso, Inc. (approved in June 2022). Additionally, Roche has submitted applications for market approval of two bispecific antibodies in China, i.e., Columvi (Glofitamab) and Vabysmo (faricimab-svoa). Akeso, Inc. has also filed for the market approval of Ivonescimab (AK112/SMT112) in China.
In addition, according to Pharmacodia Database, there are more than 10 bispecific antibodies in the world in Phase III clinical trials, more than 100 in Phase II, and more than 200 in Phase I. In terms of targets, CD3, PD-L1, PD-1, CD47, etc., are all popular targets of bispecific antibodies.
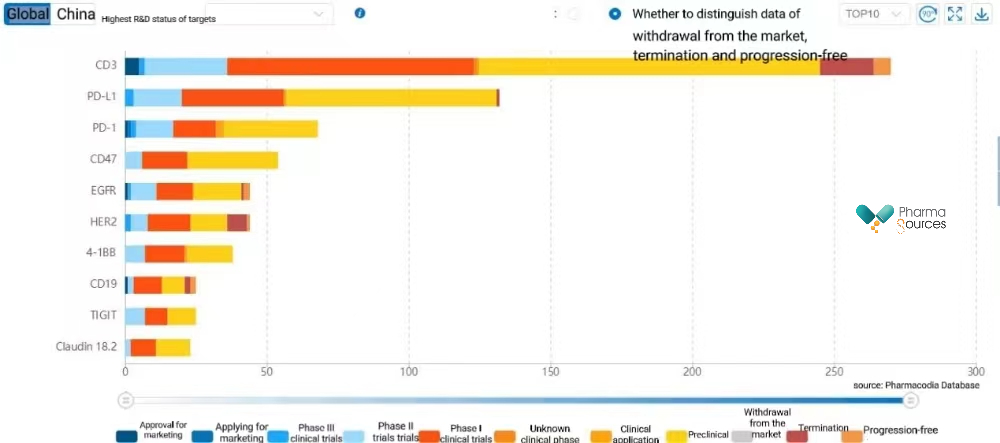
It is true that there are over 300 bispecific antibodies currently in clinical trial phases, but the number of bispecific antibodies targeting at PD-1/CTLA-4 is relatively small. In addition to QL1706 from Qilu Pharmaceutical, there are Volrustomig from AstraZeneca and Lorigerlimab from MacroGenics. Volrustomig is undergoing evaluation in five Phase III clinical trials to assess its efficacy and safety in treating various types of cancers, including NSCLC. Lorigerlimab is currently in Phase II clinical trials. Preliminary data from a Phase I clinical trial evaluating the use of lorigerlimab in the treatment of metastatic castration-resistant prostate cancer (mCRPC) showed that 28.6% (12 out of 42) of patients achieved PSA50, 21.4% achieved PSA90, and 25.7% with measurable lesions (9 out of 35) achieved a confirmed PR.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


