PharmaSources/YefenghongAugust 28, 2023
With the release of semi-annual reports of pharmaceutical companies in succession, the top 10 best-selling drugs in the world in H1 of 2023 have been unveiled. Among them, Dupixent (Dupilumab), a drug star product in the autoimmunity field of Sanofi/Regeneron, ranked sixth with sales volume of EUR 4.878 billion (around USD 5.35 billion), a year-on-year increase of 36.7% (CER). There is no suspense that it will exceed USD 10 billion at the end of the year, showing a super bombshell style.
As a fully humanized monoclonal antibody, Dupixent can restrain the signal transduction of IL-4 and IL-13 pathway, targeting at patients with type-2 inflammation. Dupixent was first approved in 2017 for the treatment of atopic dermatitis, followed by approvals for indications including asthma, chronic rhinosinusitis with nasal polyps, prurigo nodularis and eosinophilic esophagitis.
In China, Dupixent received approval for the treatment of moderate and severe atopic dermatitis in adults in June 2020. At the end of December 2020, Dupixent was officially listed in the National Catalog of Medicines for Basic Medical Insurance, Work-Related Injury Insurance, and Maternity Insurance (2020 Edition) through the national medical insurance negotiation. In September 2021, Dupixent was approved in China for the treatment of moderate and severe atopic dermatitis in adolescents aged 12 and above and adults who are in poor control or not recommended with external drugs. In February 2022, Dupixent received NMPA approval for the treatment of moderate and severe atopic dermatitis in children aged 6 and above and adults who are in poor control or not recommended with external drugs.
Under the influence of Dupixent, there is a R&D boom of IL-4 targets in China.
The immune system acts as a natural barrier of the human body. In the human immune system, after stimulation, primitive T cells can differentiate into helper T cells (Th) which have different pathways, and different forms of inflammation responses can be divided based on the cytokine groups secreted by Th cells. In which, Th2-mediated type-2 inflammation has been shown to play a main role in the barrier immunity on the mucosal surface. Th2 mainly works by secreting cytokines IL-4, IL-5, IL-9 and IL-13. IL-4 is a key cytokine, which can induce primitive CD4 + T cells to differentiate into Th2 cells, which can in turn generate more cytokines and cause type-2 inflammation response.
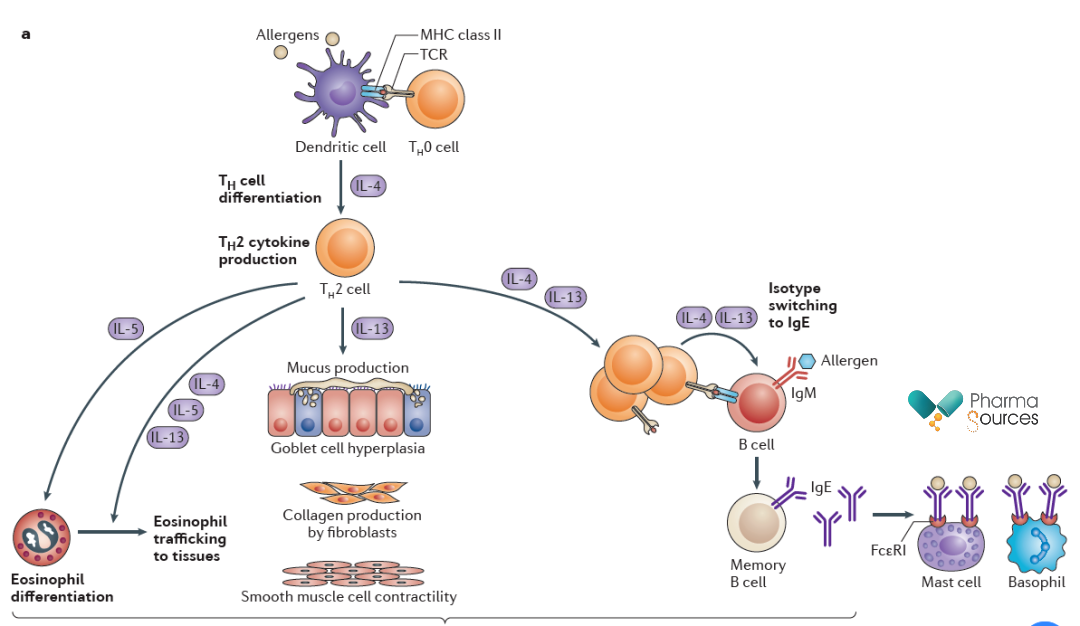
Mechanism of Action of IL-4 in Type-2 Inflammation (Source: Reference 1)
Studies have shown that IL-4 exerts biological activity by binding to its specific receptor (IL-4R). There are two types of IL-4R. Type 1 receptor, which can specifically bind to IL-4, consists of IL-4Rα subunit and γc subunit, and it is usually located on the surface of T cells, B cells, eosinophils, etc. Type 2 receptor, which can bind to IL-4 and IL-13 simultaneously, consists of IL-4Rα subunit and IL-13Rα1 subunit, and it is mostly located in epithelial cells, smooth muscle cells, etc. The binding of IL-4 to the extracellular domain of IL-4Rα brings about the conformational change of the intracellular receptor domain, activating the receptor-related Janus kinase and resulting in the recruitment and phosphorylation of STAT6. Activated STAT6 forms a homodimer, forming the translocation to the cell nucleus and facilitating the transcription of genes responsive to IL-4. Other phosphorylated tyrosine residues bind to proteins possessing the structural domain of phosphotyrosine binding (PTB), including insulin receptor substrate (IRS) proteins. The phosphorylated IRS proteins can activate PI3K/AKT signaling pathway or MAPK cascade reaction, thus activating a series of biological functions.
In addition to Dupilumab, there are a number of new drugs targeting IL-4R under research, many of which are from Chinese pharmaceutical companies.
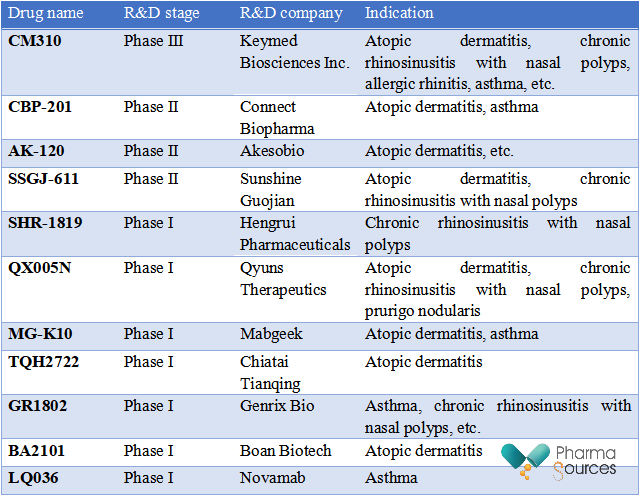
IL-4R Targeted Drugs under Research in China (Source: Public Information)
Self-developed by Keymed Biosciences Inc., CM310 is a recombinant humanized monoclonal antibody targeting at IL-4Rα and restrains biological activity by inhibiting the binding of IL-4Rα to IL-4 and IL-13. Now, the indications of CM310 for treating moderate and severe atopic dermatitis in adults have been recognized as a breakthrough therapy designation drug by CDE. In addition to atopic dermatitis, CM310 is effective for many immune diseases, including moderate and severe asthma, chronic rhinosinusitis with nasal polyps (CRSwNP), allergic rhinitis, and chronic obstructive pulmonary disease.
Clinical data on Phase Ib/IIa of moderate and severe specific dermatitis in adults indicate that after 43 days of treatment, EASI (eczema area and severity index) of 77.8% patients in high dose group decreased by 75% compared with the baseline, while only 10% in the placebo group reached EASI-75. After 43 days of high-dose CM310 treatment, 33.3% of patients had an IGA score of 0 or 1 and met an IGA score reduction of 2 points. In terms of safety, 31.0% of patients in CM310 treatment group had mild or moderate treatment-related adverse reactions, but there was no moderate or above treatment-related adverse reactions. Therefore, the safety was satisfactory.
CBP-201 is an antibody drug targeting at IL-4Rα. A Phase II clinical research conducted in the treatment of patients with moderate and severe atopic dermatitis indicated that CBP-201 reached the main effectiveness endpoint and significantly improved the percentage decline of EASI from baseline to the 16th week. All three CBP-201 treatment groups (300mg/Q2W, 150mg/Q2W or 300mg/Q4W) significantly outperformed the placebo group at the 16th week.
As a recombinant humanized monoclonal antibody targeting at human IL-4Rα and independently developed by Hengrui Pharmaceuticals, SHR-1819 can block the interleukin pathway and restrain the transmission of downstream inflammatory signals, finally improving the inflammatory state and inhibiting the progression of diseases. As SHR-1819 can block the signal transduction of IL-4 and IL-13 at the same time, it is intended for the treatment of type-2 inflammation-related diseases. In September 2021, the application of SHR-1819 for atopic dermatitis was approved by the National Medical Products Administration.
In May 2023, Hengrui Pharmaceuticals received the Notice of Approval for Clinical Trials approved and issued by CDE, allowing it to carry out clinical trials of SHR-1819 for patients with chronic rhinosinusitis with nasal polyps.
The final trial of the R&D of a drug is its clinical value. It is supposed to meet unfulfilled clinical needs. The R&D of drugs targeting at IL4Rα is expected to show better clinical data to benefit people with inflammatory diseases.
Gandhi NA,et al(2016).Targetingkey proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. doi: 10.1038/nrd4624.
Keegan AD, Leonard WJ, Zhu J. Recent advances in understanding the role of IL-4 signaling. Fac Rev. 2021 Sep 7;10:71. doi: 10.12703/r/10-71.
Shi JB, Fu QL, Zhang H, et al. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015; 70(5): 533-539.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


