Daivd Orchard Webb/PharmaSourcesJuly 03, 2023
Tag: immunotherapy , Clinical Trials , pharmaceutical companies , mRNA Vaccines
Melanoma is a type of skin cancer that develops from pigment-producing cells called melanocytes. These cells are responsible for producing melanin, which gives color to the skin, hair, and eyes. Melanoma occurs when melanocytes undergo genetic mutations and begin to grow uncontrollably. It is most commonly found on areas exposed to the sun, such as the face, neck, arms, and legs. However, melanoma can also develop in other parts of the body.
Cutaneous melanoma is staged based on several factors, including the thickness and depth of the tumor, the presence of ulceration, the involvement of nearby lymph nodes, and the presence of distant metastasis. The staging system commonly used for cutaneous melanoma is the American Joint Committee on Cancer (AJCC) TNM staging system. It classifies melanoma into stages ranging from 0 to IV, with subcategories within each stage.
The stage when melanoma is detected has a large influence on survival. The overall 5-year survival rate for cutaneous melanoma is approximately:
Stage 0 (melanoma in situ): Nearly 100% survival rate.
Stage I: Around 98% survival rate.
Stage II: Approximately 86% to 93% survival rate.
Stage III: Approximately 48% to 69% survival rate.
Stage IV (metastatic melanoma): Approximately 25% to 27% survival rate.
If melanoma is suspected, a dermatologist will perform a thorough examination and may recommend a skin biopsy to confirm the diagnosis. Treatment options for melanoma depend on various factors, such as the stage of cancer, the depth of tumor invasion, and the presence of metastasis. Treatment can involve surgical removal of the tumor, radiation therapy, immunotherapy, targeted therapy, or chemotherapy.
Cutaneous melanoma is the most common type of melanoma and develops on the skin's surface.
Uveal melanoma, also known as ocular or intraocular melanoma, develops in the eye. It affects the melanocytes within the uvea, which is the middle layer of the eye consisting of the iris, ciliary body, and choroid. Unlike cutaneous melanoma, uveal melanoma is not directly associated with sun exposure.
Cutaneous and uveal melanoma are distinct entities, and the treatment and management of each type is different. This article is limited to the discussion of treatments for cutaneous melanoma.
There are several drugs available on the market for the treatment of cutaneous melanoma. (NCCN, 2023) The choice of treatment depends on various factors such as the stage of the cancer, the presence of specific genetic mutations, and individual patient characteristics. Here are some of the commonly prescribed drugs for cutaneous melanoma:
Pembrolizumab (Keytruda) - anti-PD-1
Nivolumab (Opdivo) - anti-PD-1
Ipilimumab (Yervoy) - anti-CTLA4
Opdivo/Yervoy combination - anti-PD-1 x anti-CTLA4
Keytruda/low-dose Yervoy combination - anti-PD-1 x anti-CTLA4
Nivolumab/relatlimab (Opdualag) - anti-PD-1 x anti-LAG3
Pembrolizumab/Lenvatinib - anti-PD-1 x VGFR1/2/3
Immune checkpoint inhibitor antibodies work by blocking proteins that inhibit the immune system, thereby allowing the immune system to recognize and attack cancer cells. These immunotherapy drugs can be used alone or in combination and have shown significant efficacy in advanced melanoma.
Talimogene laherparepvec (T-VEC, Imlygic):
Imlygic/Yervoy combination
Imlygic is an oncolytic viral immunotherapy that is directly injected into the tumor to cause its destruction through both lytic and immunogenic mechanisms.
Vemurafenib (Zelboraf) - BRAF
Dabrafenib (Tafinlar) - BRAF
Encorafenib (Braftovi) - BRAF
Trametinib (Mekinist) - MEK1/2
Cobimetinib (Cotellic) - MEK
Binimetinib (Mektovi) - MEK
Dabrafenib/Trametinib combination - BRAF x MEK1/2
Vemurafenib/Cobimetinib combination - BRAF x MEK
Vemurafenib/Cobimetinib + Atezolizumab - BRAF x MEK x PD-L1
Encorafenib/Binimetinib combination - BRAF x MEK
These drugs target specific genetic mutations in the Mitogen-Activated Protein Kinases (MAPKs) BRAF and MEK, which are found in about half of all cutaneous melanomas. By inhibiting these kinases, these drugs can help slow down the growth and spread of melanoma cells.
Imatinib (Gleevec) for tumors with activating mutations of KIT
Larotrectinib for NTRK gene fusion-positive tumors
Entrectinib for NTRK gene fusion-positive tumors
Targeted therapies are designed to specifically target certain molecules or pathways involved in the growth and spread of cancer cells, including melanoma. They work by interfering with the activity of specific proteins or enzymes that are crucial for cancer cell survival and proliferation.
Interferon-alpha (INF-ɑ)
High-dose Interleukin-2 (IL-2)
These therapies are used in the adjuvant setting after surgery to reduce the risk of recurrence in high-risk melanoma patients.
Dacarbazine
Temozolomide
Paclitaxel
Albumin-bound (nab)-paclitaxel (Abraxane)
Paclitaxel/carboplatin combination
Cisplatin/vinblastine/dacarbazine (CVD) combination
There is no single standard chemotherapy regimen for cutaneous melanoma, and the specific drugs used may vary depending on factors such as the stage of the disease, individual patient characteristics, and the presence of any genetic mutations. In many cases, cytotoxic chemotherapy drugs for cutaneous melanoma are used in combination with other chemotherapy agents or with other treatment modalities like immunotherapies or targeted therapies.
Figure 1 shows the timeline of FDA drug approvals for cutaneous melanoma. The latest approval was in March 2022 for Opdualag which is a premixed combination of nivolumab (anti-PD-1) and relatlimab (anti-LAG-3) that is prepared and given through intravenous (IV) infusions. Opadualag is the only LAG-3-based checkpoint inhibitor approved for melanoma treatment.
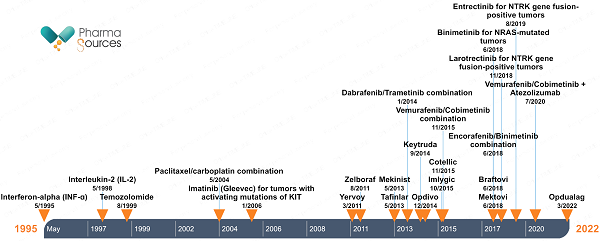
Figure 1: Timeline of FDA marketing approvals of drugs for cutaneous melanoma


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


