Daivd Orchard Webb/PharmaSourcesJuly 03, 2023
Tag: Diabetes , Metabolic Disorder , Pharmaceutical Companies
Diabetes is a chronic medical condition characterized by high levels of glucose in the blood. It occurs when the body either doesn't produce enough insulin or cannot effectively use the insulin it produces. Insulin is a hormone produced by the pancreas that helps regulate blood glucose levels and allows cells to utilize glucose for energy.
Type 1 Diabetes: In this autoimmune disease, the immune system mistakenly attacks and destroys the insulin-producing beta cells in the pancreas. As a result, the body produces little to no insulin. Type 1 diabetes typically develops in childhood or early adulthood. People with type 1 diabetes require daily insulin injections or the use of an insulin pump to manage their blood glucose levels.
Type 2 Diabetes: This form of diabetes is characterized by insulin resistance, where the body's cells become resistant to the effects of insulin. Initially, the pancreas tries to compensate by producing more insulin, but over time, it may not be able to keep up with the increased demand. Type 2 diabetes is often associated with lifestyle factors such as obesity, physical inactivity, and poor dietary habits. It typically develops in adulthood, although it is increasingly being diagnosed in children and adolescents. Type 2 diabetes can often be managed with lifestyle modifications, such as healthy eating, regular physical activity, and sometimes oral medications or insulin.
Metabolic disorder refers to a broad category of conditions that affect the body's metabolism, including the way the body processes and utilizes nutrients. Diabetes is considered a metabolic disorder because it involves abnormalities in glucose metabolism. Other examples of metabolic disorders include metabolic syndrome, which is a cluster of conditions such as obesity, high blood pressure, high cholesterol, and insulin resistance, as well as disorders of lipid metabolism, such as dyslipidemia or hyperlipidemia.
GLP-1, GIP, glucagon, DPP-4, and insulin play important roles in energy metabolism and diabetes, which is characterized by low levels of insulin (Figure 1).
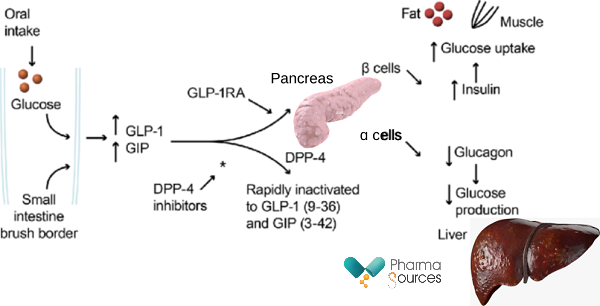
Figure 1: Mechanism of Action of GLP-1/GIP RA (Receptor Agonists) and DPP-4 inhibitors. DPP-4, inhibitors dipeptidyl peptidase-4 inhibitors; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1. Adapted from Tibaldi et al. 2014.
GLP-1 (glucagon-like peptide-1) and GIP (glucose-dependent insulinotropic peptide) are both incretin hormones produced by the gut in response to food intake. They play important roles in regulating glucose metabolism and insulin secretion.
When food is ingested, GLP-1 and GIP are released from the gut into the bloodstream. GLP-1 acts on the GLP-1 receptors located on pancreatic beta cells, promoting insulin secretion in a glucose-dependent manner. In other words, GLP-1 stimulates insulin release when blood glucose levels are elevated but has minimal effect when glucose levels are low. Additionally, GLP-1 inhibits glucagon secretion from pancreatic alpha cells, which helps to suppress the production of glucose by the liver. GLP-1 also slows down gastric emptying, which contributes to a decrease in postprandial glucose levels.
Similarly, GIP acts on GIP receptors on pancreatic beta cells, stimulating insulin secretion in response to elevated blood glucose levels. GIP also inhibits glucagon release from pancreatic alpha cells, helping to prevent excess glucose production. However, the effect of GIP on glucagon secretion is generally considered less potent compared to GLP-1.
Glucagon is a hormone produced by pancreatic alpha cells and has the opposite effect of insulin. It raises blood glucose levels by promoting glycogenolysis (breakdown of glycogen stored in the liver) and gluconeogenesis (production of glucose from non-carbohydrate sources). However, GLP-1 and GIP inhibit glucagon secretion, which helps to maintain glucose homeostasis.
DPP-4 (dipeptidyl peptidase-4) is an enzyme that degrades GLP-1 and GIP, thereby regulating their activity. DPP-4 inhibitors, which are a class of medications used to treat type 2 diabetes, inhibit the degradation of GLP-1 and GIP, leading to increased levels of these hormones in the bloodstream. By inhibiting DPP-4, these medications enhance the glucose-dependent insulin secretion and suppress glucagon release, helping to lower blood glucose levels.
Glucose is the primary source of energy for the body's cells. After a meal, the rise in blood glucose levels triggers the release of insulin from pancreatic beta cells. Insulin helps to facilitate the uptake of glucose into cells, particularly in muscle and adipose tissue, thereby reducing blood glucose levels. Insulin also promotes glycogen synthesis in the liver and skeletal muscles, storing excess glucose for later use.
Normally, glucose is filtered by the kidneys and excreted in the urine. However, SGLT-2 (sodium-glucose co-transporter 2) actively reabsorbs glucose from the tubular fluid and transports it back into the bloodstream. By reabsorbing glucose, SGLT-2 helps maintain blood glucose levels within a normal range by preventing excessive glucose loss in the urine.
In summary, GLP-1 and GIP stimulate insulin secretion while inhibiting glucagon release, helping to regulate glucose levels. Glucagon, on the other hand, raises blood glucose levels. DPP-4 regulates the activity of GLP-1 and GIP by degrading them. Insulin facilitates glucose uptake into cells and promotes glycogen synthesis. SGLT-2 maintains blood glucose levels by preventing excess secretion in the urine.
Insulin was first marketed for the treatment of diabetes in 1922. The breakthrough discovery of insulin as a life-saving treatment for diabetes was made by Sir Frederick Banting, Charles Best, and their colleagues in the early 1920s at the University of Toronto in Canada.
Since then, various forms of insulin have been developed to improve its effectiveness and duration of action. The types of insulin available today can be broadly classified into four categories:
Rapid-acting insulin: This type of insulin begins to work quickly, typically within 15 minutes after injection, and reaches its peak effect within 1 to 2 hours. It is commonly used to manage postprandial (after-meal) glucose spikes. Examples of rapid-acting insulin include insulin lispro, insulin aspart, and insulin glulisine.
Short-acting insulin: Also known as regular or neutral insulin, short-acting insulin takes effect within 30 minutes to 1 hour after injection, with a peak effect occurring in 2 to 3 hours. It can last in the body for up to 6 to 8 hours. Regular insulin is often used to manage mealtime glucose control.
Intermediate-acting insulin: This type of insulin has a slower onset of action and a longer duration compared to rapid-acting and short-acting insulins. Intermediate-acting insulin typically begins working within 1 to 2 hours, peaks in 4 to 6 hours, and lasts for about 12 to 18 hours. NPH (Neutral Protamine Hagedorn) insulin is an example of intermediate-acting insulin.
Long-acting insulin: Long-acting insulins have a prolonged duration of action and provide a steady level of insulin throughout the day, without pronounced peaks. They are used to provide basal (background) insulin coverage. There are various types of long-acting insulins available, including insulin glargine, insulin detemir, and insulin degludec. These insulins can provide glucose control for up to 24 hours or longer.
In addition to these basic categories, there are also premixed insulins available that combine both rapid-acting and intermediate-acting or long-acting insulin in a single formulation. These premixed insulins offer both basal and prandial (mealtime) coverage in one injection.
While insulin is a crucial hormone in managing blood glucose levels, there are some disadvantages to relying solely on insulin as a treatment for type 2 diabetes. This has led to the emergence of alternative targets and approaches in the management of the condition. Here are some of the disadvantages of targeting insulin:
Hypoglycemia risk: Insulin therapy carries a risk of hypoglycemia, which occurs when blood glucose levels drop too low. Hypoglycemia can cause symptoms such as dizziness, confusion, shakiness, and, in severe cases, loss of consciousness or seizures. Achieving tight glucose control with insulin may increase the risk of hypoglycemia, which can be problematic, especially for older individuals or those with conditions that impair their ability to recognize or manage low blood sugar episodes.
Weight gain: Insulin therapy is often associated with weight gain. Insulin helps transport glucose into cells, which can promote fat storage. Some individuals on insulin therapy may experience weight gain, which can further complicate diabetes management, as excess weight can contribute to insulin resistance and other metabolic issues.
Injection requirement: Insulin is typically administered via subcutaneous injections. For some individuals, the need for regular injections can be burdensome, inconvenient, or undesirable. It can be a source of discomfort, and the injection sites may develop localized reactions or irritation over time.
Progressive nature of the disease: In type 2 diabetes, insulin resistance tends to worsen over time, which means higher doses of insulin may be required to achieve glycemic control. Eventually, some individuals with type 2 diabetes may require insulin in addition to other medications or treatments to maintain glucose control.
Given these disadvantages, researchers have explored alternative targets and therapeutic approaches in the treatment of type 2 diabetes. Metformin is an oral medication commonly prescribed for the management of type 2 diabetes. It is one of the most widely used antidiabetic drugs worldwide. Metformin was first approved for the treatment of type 2 diabetes in the United Kingdom in 1957. Since then, it has been approved and used in many countries globally. The exact mechanism of action of metformin is not fully understood, but it is believed to work through several mechanisms involving reducing hepatic glucose production, improving insulin sensitivity, and modulating gut hormone responses. By targeting these processes, metformin helps lower blood glucose levels and improve glycemic control in people with type 2 diabetes.
Sulfonylureas are a class of oral medications commonly used in the treatment of type 2 diabetes. They work by stimulating insulin secretion from the pancreatic beta cells, by binding to and inhibiting ATP-sensitive potassium (KATP) channels. The first sulfonylurea approved by the FDA for the treatment of type 2 diabetes was tolbutamide in 1959. It was the first oral medication in the sulfonylurea class to be available for clinical use in the United States.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


