Dupeipei/PharmaSourcesJuly 03, 2023
Tag: Venetoclax , BCL-2 inhibitor
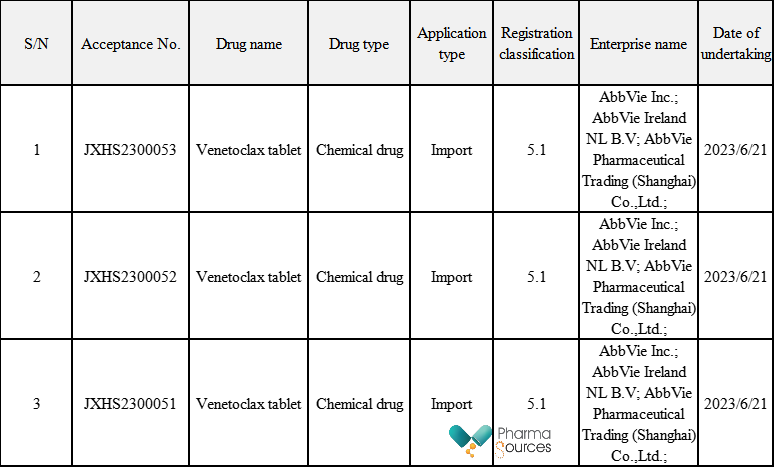
On June 21, Class 5.1 import application for 「Venetoclax tablet」 submitted by AbbVie was accepted by the CDE. Venetoclax tablet (VENCLEXTA ®), the first approved B-cell lymphoma-2 (BCL-2) inhibitor in China, was conditionally approved by NMPA in December, 2020 for treating the adult patients with Acute Myelocytic Leukemia (AML) who are not suitable for intensive induction chemotherapy due to complications or who are newly diagnosed at the age of 75 or older in combination with Azacitidine.
BCL-2 plays an important role in cell apoptosis (programmed cell death), which can prevent some cells (including lymphocytes) from apoptosis. And it can overexpress in some cancers, which is related to the drug resistance. Venetoclax makes cancer cells self-destruct to treat tumor by inhibiting the function of BCL-2 selectively and restoring the cell communication system.
At present, Venetoclax has been approved for several indications in America: Monotherapy for chronic lymphocytic leukemia (CLL) patients with 17p deletion mutation who have received at least one therapy before (FDA 2016/04); It combines with Rituxan to make a treatment for CLL or small lymphocytic lymphoma (SLL) patients with or without 17p deletion mutation who have received at least one therapy before (FDA 2018/06); It combines with a hypomethylated preparation (Azacitidine [AZA] or Decitabine [DAC]) or low dose Cytarabine (LD-AC) to make the first-line treatment for the newly diagnosed elderly AML patients at the age of 75 or older, or the adult AML patients who are not suitable for intensive induction chemotherapy due to other diseases at the same time (FDA 2018/11); It combines with Gazyva (Obinutuzumab) to make a treatment for adult CLL patients without any treatment before (FDA 2019/05).
In addition, in August 2022, the fixed treatment scheme of Venetoclax combined with Ibrutinib was approved by the EU for treating the adult CLL patients who are not treated before.
Venetoclax was developed by AbbVie and Roche, both of which are jointly responsible for its commercialization in the American market (Brand name: Venclexta), in which AbbVie is responsible for the commercialization of markets outside the America (Brand name: Venclyxto).
Venetoclax has a good market. According to the financial report of AbbVie, from 2018 to 2022, the sales volume of Venclexta is USD 344 million, USD 792 million, USD 1.337 billion, USD 1.82 billion and USD 2.009 billion respectively.
In China, Venetoclax is only approved for combining with Azacitidine to treat the newly diagnosed adult AML patients at the age of 75 or older or who are not suitable for intensive induction chemotherapy due to complications, and the above indication has entered the national medical insurance in 2022.
In addition to AML, Venetoclax has also been developed to treat recurrent or refractory CLL/SLL, recurrent or refractory multiple myeloma, myelodysplastic syndromes (MDS) and other diseases in China. The indication of Venetoclax which is declared this time in China is not yet clear, but this is one step closer to expanding the indication populations in China.
At present, only one BCL-2 inhibitor, Venetoclax, has been approved for marketing in global. In addition, many BCL-2 inhibitors under research in global have entered the clinical trial stage, as shown in the table below. Among them, AbbVie's Navitoclax is in a relatively fast progressing and has been in Phase III clinical trials.
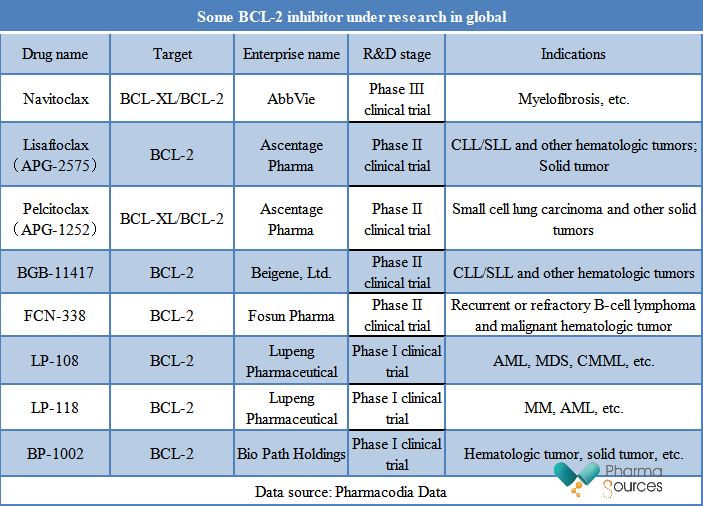
Navitoclax is the first oral BCL-XL/BCL-2 inhibitor. Phase II REFINE research data of navitoclax in the treatment of myelofibrosis (MF) published at the 2022 EHA conference show that: When navitoclax combines with JAK inhibitor ruxolitinib to treat the MF patients who have not received JAK inhibitor treatment before, their spleen volume and symptoms are improved.
Some BCL-2 inhibitors are in Phase II clinical trial, such as Lisaftoclax, Pelcitoclax and so on. Among them, Lisaftoclax (APG-2575) is a BCL-2 selective inhibitor, which is the second one in the world and the first one in China to enter into the critical registered clinical stage and has a definite efficacy. And it is carrying out clinical trials involving indications of multiple hematologic tumors and solid tumors in the world. Compared with Venetoclax, APG-2575 has a shorter half-life, a less drug exposure, and a better safety, which can potentially reduces the risk of tumor lysis syndrome. The research data of APG-2575 in patients with R/R CLL/SLL in China published at the 2023 ASCO conference show that: APG-2575 monotherapy shows a good safety in all three dose groups. And the preclinical research data published at the 2023 AACR conference show that: APG-2575 combined with Olverembatinib has a synergistic anti-tumor effect in the treatment for gastrointestinal stroma tumors (GIST) with an Imatinib-resistant, which is expected to bring a new treatment strategy to GIST patients who have drug resistance after receiving Imatinib or other TKI drugs.
Pelcitoclax (APG-1252), as a novel high-efficiency small molecule drug self-developed by Ascentage Pharma, can repair the cell apoptosis by inhibiting the BCL-2 and Bcl-xL protein selectively. In the preclinical tumor models, APG-1252 has an anti-tumor activity against a wide range of tumors, including small cell lung carcinoma (SCLC), lymphoma, colon cancer (CRC) and metastatic breast cancer (MBC).
BGB-11417 is a novel BCL-2 inhibitor with a strong effect and high selectivity self-developed by BeiGene, which potency is more than 10 times that of Venetoclax in the biochemical test. Early data of BGB-11417-101 test published at the 2022 ASH conference show that: For the R/R CLL patients, overall response rate (ORR) is 67% for BCB-11417 monotherapy and 95% for combined therapy; ORR of combined therapy for the untreated CLL patients reaches 100%, and the median follow-up time is 13.4 months, 11.1 months and 3.5 months respectively. In addition, compared with the 200mg Venetoclax, the 40mg BGB-11417 can reduce the absolute lymphocyte count (ALC) by 90%.
FCN-338, as a BCL-2 selective small molecule inhibitor self-developed by Fosun Pharma, was approved for clinical treatment for malignant tumor of blood system in July, 2020 in China. In October, 2021, FCN-338 was approved for the second clinical trial for treating recurrent or refractory B-cell lymphoma in China. In October, 2020, Fosun Pharma announced to grant Eli Lilly the exclusive right to develop, produce and commercialize FCN-338 in other regions of the world except Chinese Mainland, Hongkong and Macau, with a high transaction amount of USD 440 million.
Lupeng Pharmaceutical also developed two kinds of BCL-2 inhibitors, among which LP-108 is a self-developed novel and high-efficiency BCL-2 selective inhibitor based on the drug design of protein crystal structure, and was approved for clinical treatment in October, 2022 for combining with the Azacitidine to treat AML, myelodysplastic syndromes (MDS) or chronic myelomonocytic leukemia (CMML).
The data published at the 2023 EHA conference show that: In the treatment of recurrent or refractory MDS, CMML or AML with LP-108 single-drug or combined with Azacitidine, the overall safety is good. And LP-108 shows an encouraging anti-tumor activity in patients with various types of B-cell NHL and patients who have received BTKi treatment before.
LP-118, as a next-generation BCL-2 inhibitor, can target BCL-2 and BCL-xL at the same time, and can overcome the resistance to the first generation inhibitor Venetoclax caused by BCL-2 mutation. Three research data published at the 2022 ASH conference show that: LP-118 can effectively inhibit multiple myeloma (MM), acute myeloid leukemia (AML) and T cell acute lymphoblastic leukemia (T-ALL), and can overcome multiple acquired drug resistance, with a strong safety.
In addition to the small molecular drug, the antisense RNAi drug is also a type of BCL-2 inhibitors under study. Among them, BP1002 is a kind of antisense RNAi drug with neutral charge and mixed with liposomes, which inhibits the BCL-2 protein synthesis by targeting BCL-2 at mRNA level instead of protein level. Preclinical research shows that BP1002 is an effective inhibitor of BCL-2, its good safety makes it take a combined treatment with approved drugs, and the combination of BP1002 and Decitabine is effective on venetoclax-resistant cells.
Since BCL-2 protein was found firstly in 1984, only one BCL-2 inhibitor has been approved for marketing through nearly 40 years of development. At present, many BCL-2 inhibitors have entered the clinical trial in global, which are mainly developed for treating the hematologic tumors. It is worth mentioning that our pharmaceutical companies also actively engage in BCL-2 inhibitor field, especially Ascentage Pharma and Lupeng Pharmaceutical. Among them, BCL-2 inhibitor of Fosun Pharma has been abroad successfully which has been licensed to Eli Lilly.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


