Jianghuzhiyuan/PharmaSourcesJune 27, 2023
Tag: HDAC inhibitor , Anti-tumor , Hematologic Tumor
Recently, Xynomic Pharmaceuticals announced that it has made key progress in clinical trials for three different indications of HDAC inhibitor with the potential of "Best in class", Abexinostat, and two of which are the best data in the world.
1. Phase I clinical trial of Abexinostat in combination with the BTK inhibitor Ibrutinib of Janssen in the treatment of mantle cell lymphoma in the United States has been successfully completed, and the complete response rate(the proportion of cases where the tumor completely disappeared) achieved was up to 71%, indicating the best complete response rate in the world.
2. The US FDA approved Abexinostat as the first-line or second-line drug in the treatment of renal cancer, which is a revision of phase 3 protocol for global registration. Previously, the Phase I clinical study data of Abexinostat in combination with Pezopanib in the treatment of renal cancer showed that the median overall survival (mOS) in patients with renal cancer is 27.65 months, which is the best data in the world for the treatment of renal cancer.
3. The Phase I clinical trial of Abexinostat in combination with Temozolomide in the treatment of recurrent glioma (GBM) has been initiated. Preclinical study has shown that Abexinostat can efficiently cross the blood-brain barrier, and the effect was promoted by Temozolomide to enhance the efficacy of Temozolomide and improve the survival rate of glioma patients.
Abexinostat is a new specific HDAC inhibitor with GBM characteristics, which is being applied for national class 1 new drug, and it is mainly used for the treatment of lymphoma and renal cancer. Xynomic Pharmaceuticals has the rights and interests in the exclusive global development, production and commercialization of Abexinostat.
Histone deacetylase (HDAC) inhibitor are a new class of drugs developed based on the theory of epigenetics. As the basic unit of chromatin of eukaryote, Nucleosome affects the function of cells by modification of the acetylation, methylation, phosphorylation and ubiquitism of the N-terminal of histone core. The dynamic equilibrium between histone acetylase (HAT) and histone deacetylases (HDAC) can control the chromatin structure and gene expression. When the deacetylation level of histone increases, the acetylation level relatively decreases, which can result in the changes in normal cell cycle and metabolic behaviors and induce tumors and neurodegeneration. HDAC inhibitor targeting HDAC can regulate the acetylation of histone, promote the transcription and expression of anti-tumor transcription factors, regulate the related signal pathways and exert anti-tumor biological effects by inhibiting the HDAC activity. Studies have shown that HDAC inhibitor can exert an anti-tumor effects by promoting cell differentiation, blocking cell cycle, inducing apoptosis, and up regulating the expression of tumor suppressor gene such as p21cip/WAF.
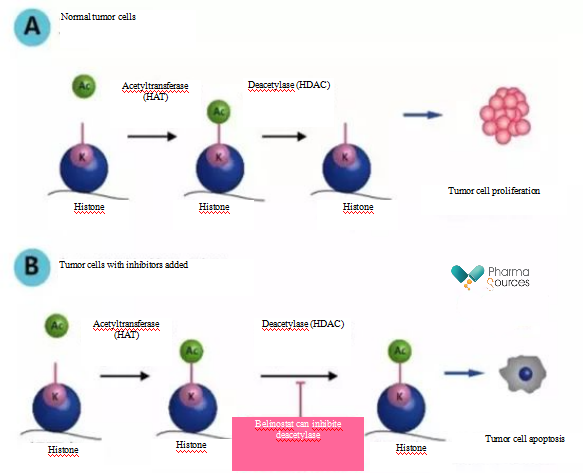
As a mature anti-tumor target, HDAC inhibitors are currently mainly used for blood diseases, and most products are undergoing clinical trials for new indications such as breast cancer, non-small cell lung cancer, pancreatic cancer. With further study, the range of indications may be increased.
Currently, there are a total of 5 HDAC inhibitors approved for market worldwide. Vorinostat, Romidepsin, Belinostat, and Panobinostat are approved by the US FDA for marketing in the clinical treatment of peripheral T-cell lymphoma, cutaneous T-cell lymphoma, and multiple myeloma; Chidamide (brand name: apsara®) is approved for marketing by NMPA in China and used for peripheral T-cell lymphoma and breast cancer. Among them, except chidamide, the Class I HDAC inhibitors and subtype selective HDAC10 inhibitors, the other four are pan-HDAC inhibitors.
There are mainly 8 drugs in the clinical stage with indications for hematologic tumor related diseases in China. As shown in Table 3, Entinostat and Abexinostat, which have made big progress, have entered clinical phase III; BEBT-908 and Purinostat are currently undergoing Phase II clinical trials.
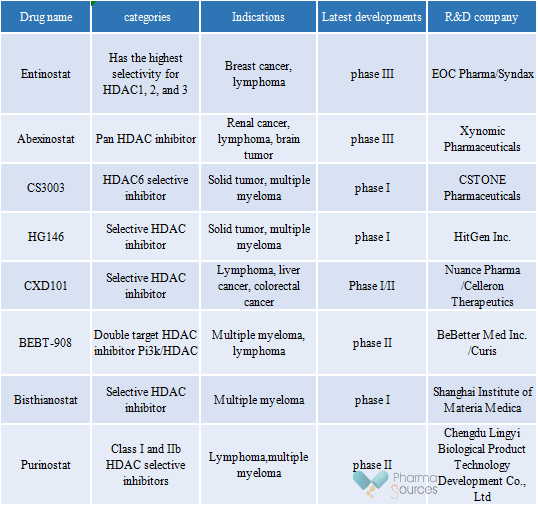
Entinostat is a new and oral HDAC inhibitor. It can improve and regulate the hyperacetylation of histone and promote the transcriptional activation of specific genes by selectively inhibiting Class I HDAC, especially the representative HDAC subtype 1, 2 and 3, and ultimately inhibit cell proliferation, accelerate terminal differentiation and/or induce apoptosis. In 2013, EOC Pharma (Hong Kong) Limited signed a licensing agreement with Syndax Pharmaceuticals (NASDAQ: SNDX) and obtained the rights and interests in R&D, production and commercialization in Chinese Mainland. In January this year, Entinostat was first applied for marketing in China for the treatment of advanced breast cancer patients with Hormone receptor (HR) positive and human epidermal growth factor receptor 2 (HER-2) negative. At present, Entinostat is only developed for treatment of breast cancer in China, but globally, it is intended to be used in the treatment of hematological malignancy (Hodgkin lymphoma, acute lymphoblastic leukemia, chronic myelogenous leukemia, etc.), colorectal cancer, cholangiocarcinoma, fallopian tube cancer, peritoneal cancer, etc. The combination of drugs that have been investigated include Keytruda, atezolizumab, enzalutamide, etc.
BEBT-908 is the first class 1 new drug of PI3K/HDAC dual target anti-tumor independently developed by BeBetter Med Inc., which can selectively inhibit the core target with synergistic effect, destroy the signal pathway network of tumor, and has a strong anti-tumor effect on a variety of hematologic tumor and solid tumors. In September 2021, it was included in the categories of groundbreaking treatments by CDE for the treatment of relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) after at least two systematic treatments.
At present, the phase IIa clinical study on the treatment of relapsed or refractory diffuse large B-cell lymphoma (r/r DLBCL) has been completed, and safe and effective data have been obtained. The phase II clinical trial of key validation is being carried out. In addition, other ongoing clinical trials of BEBT-908 include: Phase II clinical trial for the treatment of relapsed and refractory T-cell lymphoma; Phase II clinical trial for the treatment of relapsed or refractory follicular lymphoma, marginal zone lymphoma, and lymphocytes and white blood cells; three phase II clinical trials for the monotherapy of PIK3CA mutated advanced solid tumor with single drug, ER+/HER2 advanced breast cancer in combination with fulvestrant, advanced solid tumor in combination with PD1/PD-L1 monoclonal antibody.
Official website of CDE
Official website of Xynomic Pharmaceuticals
Official website of EOC Pharma
Frew AJ,Johnstone RW,Bolden JE.Enhancing the apoptotic and therapeutic effects of HDAC inhibitors[J].Cancer Lett,2009,280(2):125—133.Mantese G. Gastrointestinal stromal tumor: epidemiology, diagnosis, and treatment. Curr Opin Gastroenterol. 2019; 35(6): 555-559.
Johnstone R,Ruefli A,Lowe S.Apoptosis:a link between caneer genetics and chemotherapy[J].Cell,2002,108(2):153—164.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


