PharmaSources/zhulikou431June 12, 2023
Tag: Drug Shortage , Drug Supervision
It was recently reported that the U.S. FDA is working with Chinese drugmaker Qilu Pharmaceutical to import the anti-cancer drug cisplatin to increase supply in the face of ongoing shortages. This can be confirmed by the FDA's drug shortage database.
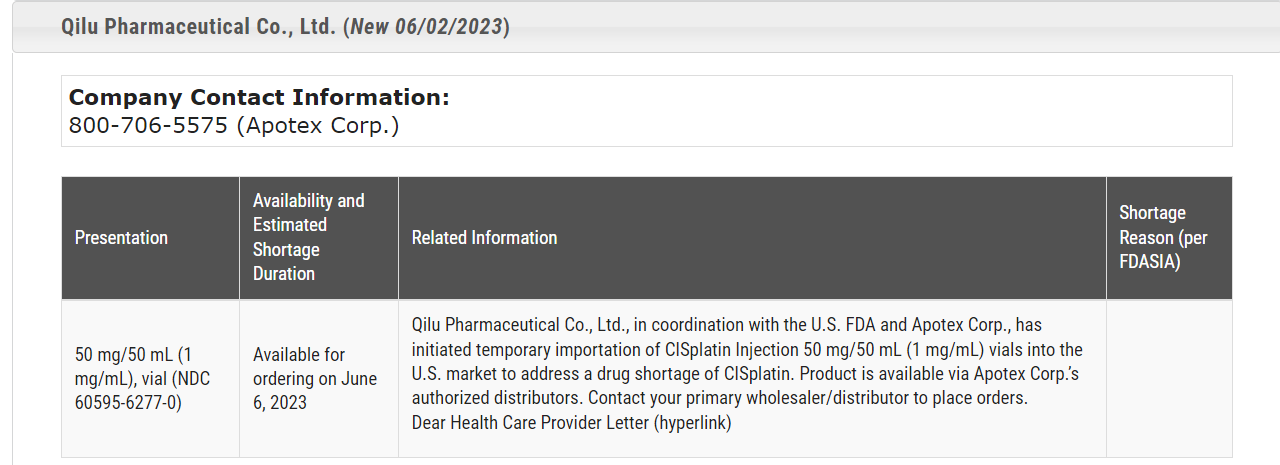
Figure 1 Information on the FDA's initiation of temporary import of Qilu cisplatin injection (from FDA's website)
Clearly, drug shortages are continuing to plague national drug authorities and governments. So far, Covid-19 has been disarmed, and global trade and business communication can be carried out smoothly. However, pressures such as cost, regulatory pressure, technological pressure and environmental protection will continue to interfere with the stable global supply of medicines. This paper will present the regulatory requirements and dynamics of drug shortages in major pharmaceutical markets around the world.
Drug shortages have been a global issue, and the U.S. has been experiencing drug shortages for many years, with more than 100 drug shortages identified in the country each year. According to the latest drug shortage database of the FDA, there are 8 new shortage drugs as of 2023 (Figure 2), including the anti-cancer drug cisplatin imported from Qilu, and most of the other new shortage drugs are due to drug discontinuation.

In addition, according to the database, there are currently 207 drugs in shortage in the U. S. (68 of which have been resolved) and 346 discontinued drugs. In addition, in its therapeutic classification, there are shortages of a variety of important drugs in the areas of cancer treatment, respiratory system, cardiovascular system, as well as anti-infective and antiviral.

There is no doubt that the U.S. is one of the most innovative countries in the healthcare industry, but the issue of continued availability of domestic drugs in the U.S. has been difficult to resolve. In the past two years, U.S. drug shortages have been near all-time highs and have affected a wide range of people due to the impact of Covid-19 and disruptions in the supply chain. For example, shortages of the most common antibiotic amoxicillin are frequently on the list, and there are also a variety of diabetes drugs and anti-cancer drugs that are not in steady supply.
The reasons for the drug shortage in the U.S. are related to the way its drugs are regulated; another important reason is that many preparations in the U.S. rely mostly on imported APIs or ingredients, and there is a big crisis in the drug supply chain. Further, the drug shortage is a result of labor shortages or product quality issues in U.S. manufacturing. Inexpensive generic drugs generate very low profits, thus leading to a continued lack of incentive for drug manufacturers to produce certain low-margin drugs.
The FDA has established direct communication channels in order to quickly gather information on drug shortages:
https://cdernextgenportal.fda.gov/Login_CDER?ec=302&startURL=%2Fs%2F
If the public has any questions, you can write to the FDA (drugshortages@fda.hhs.gov) for feedback.
On the FDA's website, the following strategies are listed as recommended to address the drug shortage:
--Reach consensus on the impact of drug shortages on patients and collaborative practices that may lead to shortages.
--- Develop a rating system to incentivize drug manufacturers to invest in the quality management maturity of their facilities.
In FY2022, the FDA launched the Quality Management Maturity (QMM) program to promote a stable supply of drugs.
--Promote sustainable private sector contracts (e.g., with payers, purchasers, and group purchasing organizations) to ensure a reliable supply of medically important drugs.
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


