Caicai/PharmaSourcesJune 06, 2023
Tag: Aripiprazole , Long-acting , Marketing
On May 23, the NMPA official website revealed that Aripiprazole (Aripiprazole long-acting intramuscular injection) developed by Otsuka Pharmaceutical to treat schizophrenia was approved for marketing. The once-a-month injection has the advantages of high availability, excellent control of hospitalization rate, good clinical compliance, and more benefits for patients compared to regular preparations.
Aripiprazole is an atypical antipsychotic, and its molecular formula is C23H27Cl2N3O2. Aripiprazole has a unique mechanism of action that can partially activate the dopamine D2 receptor and 5-serotonin 1A receptor while antagonizing the 5-serotonin 2A receptor. It is used to treat schizophrenia, acute manic episode or mixed episode, and bipolar affective disorder, which is the preferred drug recommended by the Expert Medication Guides of the United States for the treatment of first-episode or recurrent schizophrenia.
Aripiprazole was developed by Otsuka Pharmaceuticals and was approved for marketing in the United States in November 2002, December 2004, June 2006, and September 2006 as tablets, oral liquid, orally disintegrating tablets, and intramuscular injections, respectively. Its brand name is Abilify.
Aripiprazole long-acting intramuscular injection was jointly developed by Otsuka Pharmaceuticals and Lundbeck Pharmaceutical. It was approved for marketing by the FDA in February 2013 for the treatment of schizophrenia. Its brand name is Abilify Maintena, with specifications of 300 mg/bottle and 400 mg/bottle. The frequency of administration is once a month. After the first injection, patients need to additionally take the oral preparation of Aripiprazole for 14 days to maintain the effective therapeutic concentration at the initial stage.
In March 2021, Otsuka Pharmaceutical submitted a marketing application for Aripiprazole long-acting intramuscular injection in China and was accepted. The acceptance numbers were JXHS2100029 and JXHS2100030, and the indication was schizophrenia.
According to the clinical trial registration and information disclosure platform, Otsuka Pharmaceutical has conducted three clinical trials abroad, including one phase III trial.
Aripiprazole is one of the best-selling psychiatric medications, with a global peak sales volume exceeding US$8 billion and a cumulative sales volume exceeding US$63.5 billion. Since its marketing, the market sales of the long-acting formulation of Aripiprazole have maintained a positive growth rate, mainly in the United States, Japan, and Europe.
In 2021, the global sales volume of Aripiprazole long-acting intramuscular injection (brand name: Abilify Maintena) reached US$1.371 billion, a year-on-year growth of 10.74%.
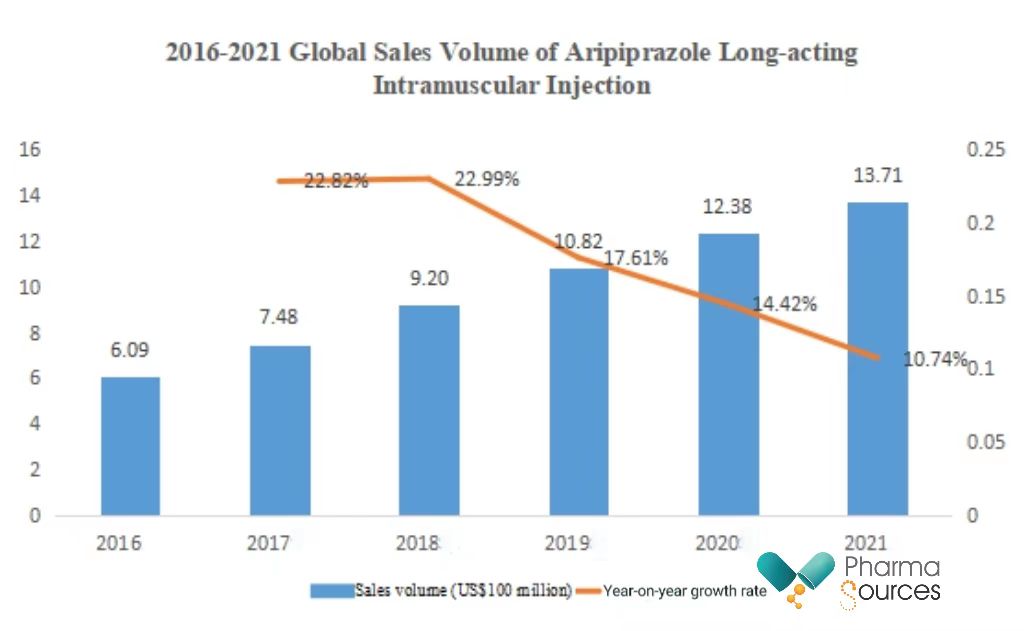
(Data source: annual reports of Otsuka Pharmaceut ical and Lundbeck Pharmaceutical)
In 2021, the sales volume of all formulations of Aripiprazole (ordinary tablets, orally disintegrating tablets, oral liquid, capsules) in China was RMB1.147 billion, a year-on-year growth of 8.11%.
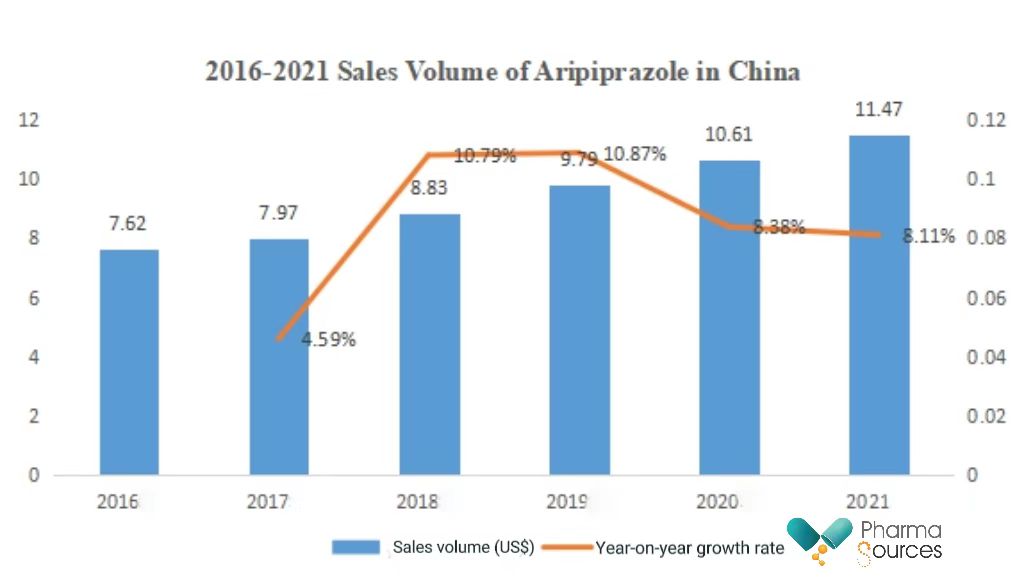
(Source: database of Yaozhi.com)
Other than Otsuka Pharmaceutical, there are another two companies in the clinical research stage in China.
The Aripiprazole long-acting intramuscular injection of Hunan Kelun Pharmaceutical Co., Ltd. was applied for a Class 3 chemical drug and obtained NMPA approval for clinical trials on October 17, 2017. The Aripiprazole microspheres for injection developed by Zhuhai Livzon Microsphere Technology Co., Ltd. were applied for a Class 2 chemical drug and obtained NMPA approval for clinical trials on April 30, 2020. Later, the drug was registered for two phase-I clinical trials on July 16, 2020 and September 16, 2021. The two are once-a-month injections.
On April 27, Aripiprazole pre-filled long-acting injection was approved by the FDA for marketing, under the brand name ABILIFY ASIMTUFII, for use in adult schizophrenia or as a single-drug maintenance therapy for patients with bipolar I disorder. This drug, jointly developed by Otsuka Pharmaceuticals and Lundbeck Pharmaceutical Lingbei, is a long-acting injection formulation pre-filled in a syringe, which does not require patients to dissolve the drug. A single intramuscular injection can provide a continuous treatment concentration of two months.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


