Dopine/PharmaSourcesJune 06, 2023
Tag: TQ-B3525 , Follicular lymphoma , PI3K inhibitor
On May 25, the CDE official website revealed that the marketing application for the first-in-class "TQ-B3525 tablets" by Chiatai Tianqing was proposed to be included in the priority review for the treatment of recurrent or refractory follicular lymphoma (FL) patients who have previously received at least two systemic therapies.
TQ-B3525 is a new phosphatidyl inositol 3-kinase (PI3K) α/δ dual inhibitor developed by Chiatai Tianqing, which inhibits the expression of PI3K protein and reduces AKT protein phosphorylation levels to induce cell apoptosis and then inhibit the proliferation of malignant tumor cells.
By selectively inhibiting PI3Kδ and PI3Kα subunits, TQ-B3525 can overcome the drug resistance problem caused by the PI3Kα up-regulating activity due to individually inhibiting PI3Kδ. Early clinical research data have confirmed the oustanding therapeuticl effect of TQ-B3525 tablets in advanced malignant tumors, such as recurrent/refractory FL.
The results of phase 1 clinical trial show that: TQ-B3525 is well tolerated in Chinese patients with advanced malignant tumors and has high anti-tumor activity in patients with recurrent or refractory lymphoma.
In July 2021, TQ-B3525 was included in the category of breakthrough therapy designation by CDE for the treatment of recurrent/refractory FL patients who have previously failed at least second-line treatment.
On May 22, Sino Biopharm announced that the phase 2 critical registered clinical trial of TQ-B3525 for the treatment of recurrent or refractory FL patients who have previously received at least second-line systemic treatment had reached the primary endpoint.
FL is the second most common non-Hodgkin's lymphoma (NHL). In China, 8.1% to 23.5% of FLs are NHL, and about 80% of the patients are in the advanced stage (III/IV) when diagnosed. Although FL is classified as indolent lymphoma, the vast majority of patients will experience multiple relapses and progression with invasive diseases, which may lead to death within 1-2 years. Currently, FL is an incurable disease, and the treatment options for recurrent/refractory FL in China are limited, with unmet clinical needs.
This proposed inclusion of TQ-B3525 in the priority review by CDE will accelerate its approval for marketing in China. We look forward to its early approval in the future to benefit FL patients.
PI3K (full name: phosphatidyl inositol 3-kinase) is an intracellular lipid phosphokinase composed of the p85 regulatory subunit, p55 regulatory subunit, and p110 catalytic subunit. Based on the different structures and substrates, PI3K can be divided into class I, class II, and class III. Class I PI3K is a heterodimer composed of PI3K catalytic subunits and regulatory subunits, which is currently the most deeply and extensively studied subtype and has the closest relationship with tumors.
Class I PI3K can be further divided into class IA and class IB by the catalytic subunits. The catalytic subunits in class IA PI3K include three proteins, i.e., p110α, p110β, and p110δ, while class IB PI3K only contains the p110γ catalytic subunit. Class IA PI3K is closely related to the occurrence and development of tumors, among which PIK3CA, the gene that compiles PI3Kα, is the most common mutation in tumors. After the mutation, PIK3CA can abnormally activate PI3Kα while inhibiting the expression of the tumor suppressor gene PTEN, and therefore PI3Kα is a vital target in drug R&D. The expression of PI3K varies among different catalytic subunits. For example, PI3Kα and PI3Kβ are expressed in multiple cells, and PI3Kδ and PI3Kγ is only expressed in the immune system.
PI3K is a key regulatory kinase in the PI3K/AKT/mTOR signaling pathway, which is involved in regulating cell proliferation, differentiation, apoptosis, and angiogenesis. Its abnormal activation is closely related to the occurrence and development of various tumors. At present, seven PI3K inhibitors have been approved globally (see table below). Among them, the approval of Ukoniq was accelerated by the FDA in June 2021 to treat marginal zone lymphoma (MZL) and FL. However, in June 2022, for safety concerns, the FDA ultimately decided to withdraw Ukoniq's marketing approval because it may lead to a higher mortality rate.
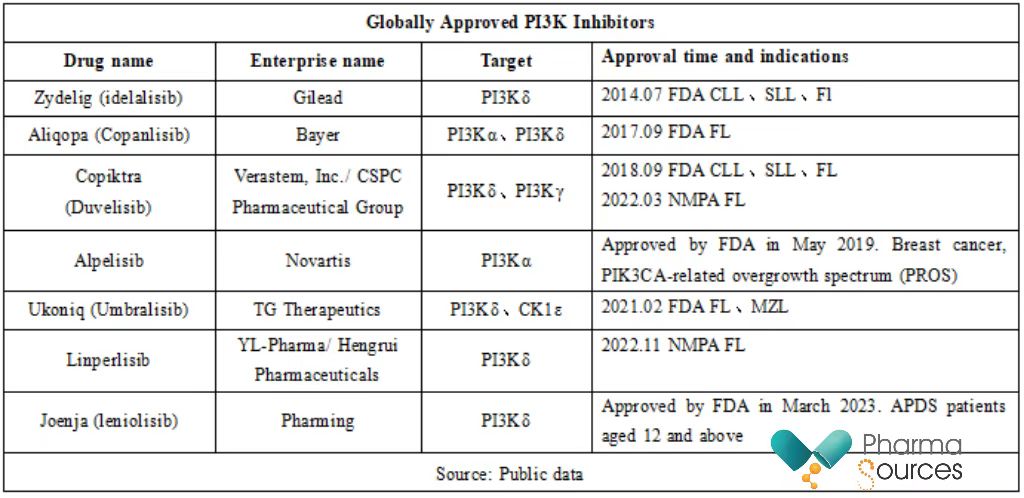
In terms of indications, only alpelisib developed by Novartis is approved for the treatment of solid tumors - HR+/HER2- locally advanced or metastatic breast cancer with disease progression after receiving endocrine therapy with monotherapy and carrying PIK3CA mutation. In addition, alpelisib passed the FDA accelerated approval in April 2022 under the brand name Vijoice to treat adults and pediatric patients aged two and above who have severe PROS manifestations and require systemic treatment. Joenja developed by Pharming was approved to treat adult/adolescent patients aged 12 and above with activated APDS. APDS is a rare primary immunodeficiency disease caused by mutations in the PIK3CD or PIK3R1 genes.
At present, China's NMPA has approved three PI3K inhibitors, namely copanlisib (approved in May 2023) developed by Bayer, duvelisib (approved in March 2022) developed by CSPC Pharmaceutical Group/Verastem, and linperlisib (approved in November 2022) developed by YL-Pharma/Hengrui Pharmaceuticals. China has approved them to treat recurrent or refractory FL patients who have undergone at least two previous systematic treatments.
In addition, there are multiple PI3K inhibitors under research worldwide, such as Inavolisib (GDC-0077) developed by Roche, gedatolisib developed by Celcuity, HMPL-689 developed by Hutchmed, and SHC014748M developed by Sanhome Pharmaceutical.
However, safety issues have always plagued the R&D of PI3K inhibitors. Among them, Idelalisib developed by Gilead has been given four black box warnings since its marketing, indicating the risk of life-threatening liver problems. In March 2016, due to serious adverse events caused by Idelalisib, such as infection-related deaths, that occurred in clinical research, it was successively warned and investigated by the EMA and FDA. Later, Gilead terminated all subsequent development plans for Idelalisib. In 2022, Gilead voluntarily withdrew Idelalisib treatment for two indications, FL and SLL.
In addition, Bayer withdrew the marketing application of Copanlisib to treat MZL in the EU. Secura Bio withdrew some approved indications of Duvelisib. TG Therapeutics voluntarily withdrew the supplemental New Drug Application (sNDA) of Umbralisib combined with Ublituximab in treating CLL and SLL and stopped selling Umbralisib. Incyte Corporation withdrew the NDA of Parsaclisib for the treatment of R/R FL, MZL, and MCL. The new drug, zandelisib, a PI3K inhibitor developed by MEI Pharma, was rejected by the FDA.
Moreover, after FDA re-examined the long-lasting side effects and safety impacts of PI3K inhibitors in April 2022, the FDA Oncologic Drugs Advisory Committee (ODAC), with 16 votes in favor, 0 negative votes, and 1 abstention, agreed to re-plan the approval framework of PI3K drug development and proposed three changes: Firstly, advocate for careful dose selection (including approved drugs) through robust dose exploration in early randomized trials; Secondly, avoid using single-arm trials as a regulatory strategy, and adopt random trials instead; Finally, comprehensively collect and analyze OS data to evaluate the impact of drugs on this 'ultimate safety endpoint'.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


