Yi/PharmaSourcesJune 06, 2023
Tag: ADC , Kelun Pharmaceutical , ADC transactions
On May 12, LaNova Medicines announced an exclusive licensing agreement with AstraZeneca for the global development and commercialization rights regarding GPRC5D targeted antibody-drug conjugate (ADC) LM-305. According to the agreement, LaNova Medicines will receive a down payment of USD 55 million and the payment for recent milestones, plus a payment for development and commercialization milestones of up to USD 545 million as well as a gradient commission based on global net sales volume.
LM-305 is the world's first ADC targeting GPRC5D entering the clinical stage. In October 2022, it was approved for clinical research in China to treat multiple myeloma (MM) and other plasma cell diseases. GPRC5D is a member of the G-protein coupled receptor (GPCR) family, which is mainly expressed in malignant bone marrow plasma cells and hair follicles but seldom or never expressed in normal tissues. Research has found that GPRC5D is specifically overexpressed in MM patients, and its expression level is relatively independent of another target BCMA for MM.
It is worth mentioning that LM-305 is the second drug developed by LaNova Medicines expanding into the world market. In May 2022, LaNova Medicines reached an exclusive licensing agreement with Turning Point USA, authorizing the global development and commercialization rights of Claudin18.2 targeting ADC LM-302 in countries and regions other than Greater China and South Korea to Turning Point. According to the agreement, Turning Point will pay LaNova Medicines a down payment of USD 25 million and the payment for R&D milestones and subsequent commercialization milestones of USD 195 million, with a total amount exceeding USD 1 billion.
With the rise of ADCs, more ADCs made in China have expanded into the world market. See the table below. Pharmacodia data reveal that there have been at least 15 overseas transactions of ADCs made in China, with a total transaction amount exceeding USD 25.122 billion. Among them, nine individual transactions exceeded USD 1 billion, and the highest amount of a single transaction reached USD 9.3 billion. In terms of R&D stage, most ADCs entering the world market are in early clinical trials, and currently only Disitamab Vedotin has been approved for marketing.
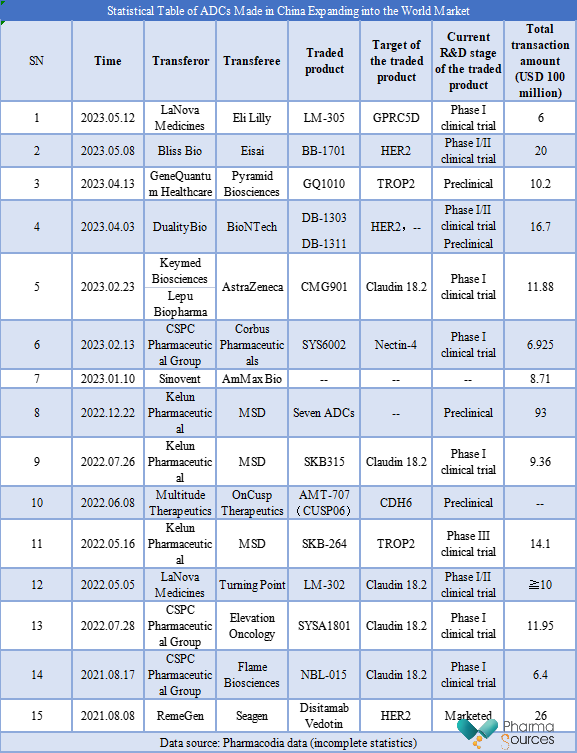
In terms of the transaction time, two, six, and seven ADC overseas transactions were concluded in 2021, 2022, and 2023, respectively. In 2023, seven transactions were completed in less than five months, exceeding the total number of ADC overseas transactions in 2022. It can be seen that the number of ADC overseas transactions has been increasing year by year.
In terms of the annual total amount of ADC overseas transactions, the year 2022 ranked first, with a cumulative amount of USD 12.841 billion. The total amount of ADC overseas transactions in less than five months in 2023 reached USD 8.0415 billion.
In terms of targets, ADCs entering the world market mainly focus on Claudin 18.2, TROP2, and HER2, which are popular targets for the R&D of ADCs made in China. Currently, some ADCs targeting TROP2 and HER2 have been approved for marketing. In addition, ADCs expanding into the world market also involve some emerging targets, such as GPRC5D and CDH6.
In terms of enterprises, Kelun Pharmaceutical, CSPC Pharmaceutical Group, and LaNova Medicines concluded most ADC transactions. Kelun Pharmaceutical completed three overseas transactions, with the highest total amount among the three, which was USD 11.646 billion. CSPC Pharmaceutical Group ranked second, which was up to USD 2.5275 billion. LaNova Medicines ranked third, completing two ADC overseas transactions with a total amount of over USD 1.6 billion. In addition, it is worth mentioning that the transferee of all three ADC overseas transactions of Kelun Pharmaceutical was MSD, while LaNova Medicines and CSPC Pharmaceutical Group made deals with different foreign pharmaceutical companies.
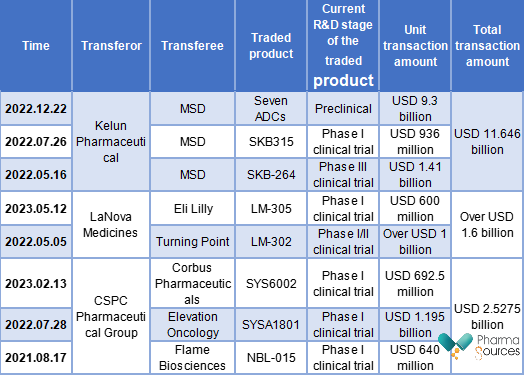
With the R&D capabilities of Chinese pharmaceutical companies, the innovation of ADCs made in China has also received attention and favor from multinational pharmaceutical companies and foreign biotech companies, which will further accelerate the internationalization process of ADCs made in China. It is noteworthy that more ADCs made in China have concluded overseas transactions in the past three years, increasing the number of transactions year by year. Remegen has indeed been the pioneer of ADCs made in China entering into the world market. However, Kelun Pharmaceutical excels in both the number and total transaction amount.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


