Caicai/PharmaSourcesMay 22, 2023
Tag: Sevelamer Carbonate Tablets , Dr. Reddy's Laboratories , Generic Drugs
On May 8, the NMPA official website revealed that the type 5.2 chemical drug, Sevelamer Carbonate Tablets, of Dr. Reddy's Laboratories had been approved for marketing in China. Previously, one original research company and three generic drug companies had been approved in Chinese market, and Dr. Reddy's Laboratories became the fourth pharmaceutical company with generic drugs approved for marketing, and also the first foreign pharmaceutical company.
In July 2019, Dr. Reddy's Laboratories submitted a marketing application of type 5.2 chemical drug, Sevelamer Carbonate Tablets, which was accepted by the CDE.
Sevelamer carbonate, also known as cross-linked polyallylamine polymer, is a copolymer of polyacrylamide and epichlorohydrin by cross-linked polymerization. This kind of drug was developed by GENZYME (acquired by Sanofi in 2011) and approved for marketing by FDA in 2007. It was approved by the NMPA in China in 2013. The preparations on the market are mainly tablets (brand name: Novella/Renvela) and dry suspension (brand name: Renagel).
Sevelamer Carbonate Tablets are the first-line drug for treating hyperphosphatemia of adult patients with chronic kidney disease (CKD). Hyperphosphatemia is a pathological state in which the serum inorganic phosphorus concentration is higher than the normal level. Kidney diseases (such as chronic kidney disease and renal failure) are the main causes.
At present, the prevalence rate of chronic kidney diseases among Chinese adults is 10.8%. The main therapeutic drugs used clinically for the treatment of hyperphosphatemia are phosphorus binders, which can be divided into four categories: I. Aluminum phosphate binders represented by aluminum hydroxide; II. Calcium phosphate binders represented by calcium carbonate and calcium acetate; III. Non-aluminum and non-calcium metal-containing phosphate binders represented by lanthanum carbonate; IV. Non-calcium and non-metallic phosphate binders represented by sevelamer.
In contrast to other phosphate binders, sevelamer carbonate does not increase risks of vascular calcification, cardiovascular or metal absorption and accumulation in patients as calcium and metal-phosphate binders do. Meanwhile, the drug is effective, which can significantly reduce the serum phosphorus level, iPTH, total cholesterol and LDL-C level of hemodialysis patients; delay the vascular calcification; and effectively reduce the risk of cardiovascular mortality. Long-term use will not trigger serious adverse reactions and the security is high.
Although the indications of Sevelamer Carbonate Tablets are small, because of the high prevalence rate of chronic kidney diseases among Chinese adults, the sales volume of this drug is still considerable. Recent statistics show that the sales volume of the drug within hospitals reached RMB 666 million in 2021.
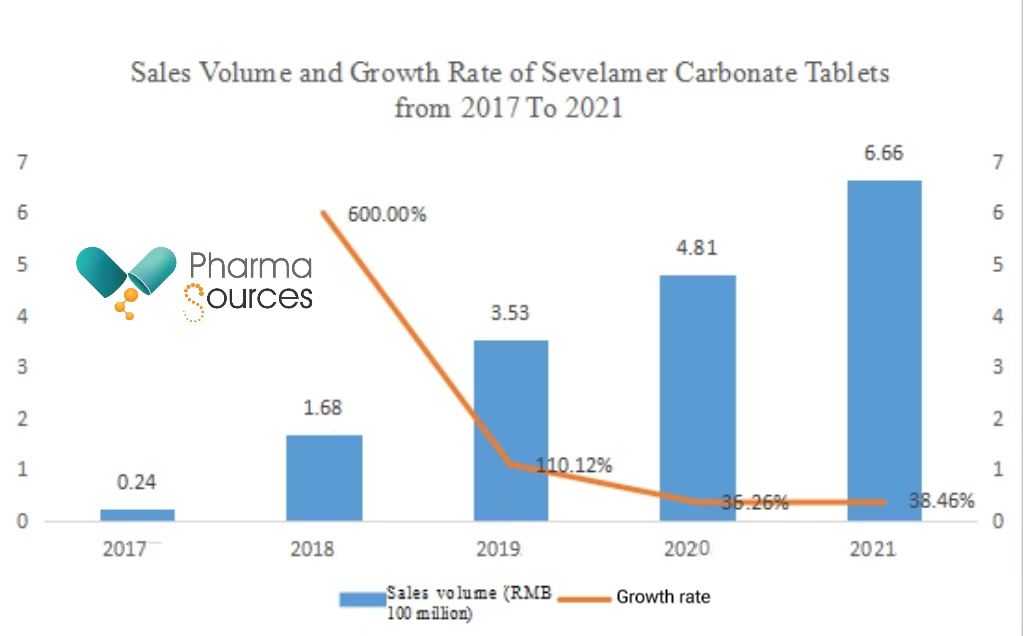
(Data source: Pharnexcloud National Pharmaceutical Sales Database)
So far, a total of 11 companies have submitted marketing applications for generic drugs of Sevelamer Carbonate Tablets in China, including 9 Chinese pharmaceutical companies and 2 foreign ones, and 4 of them have been approved.
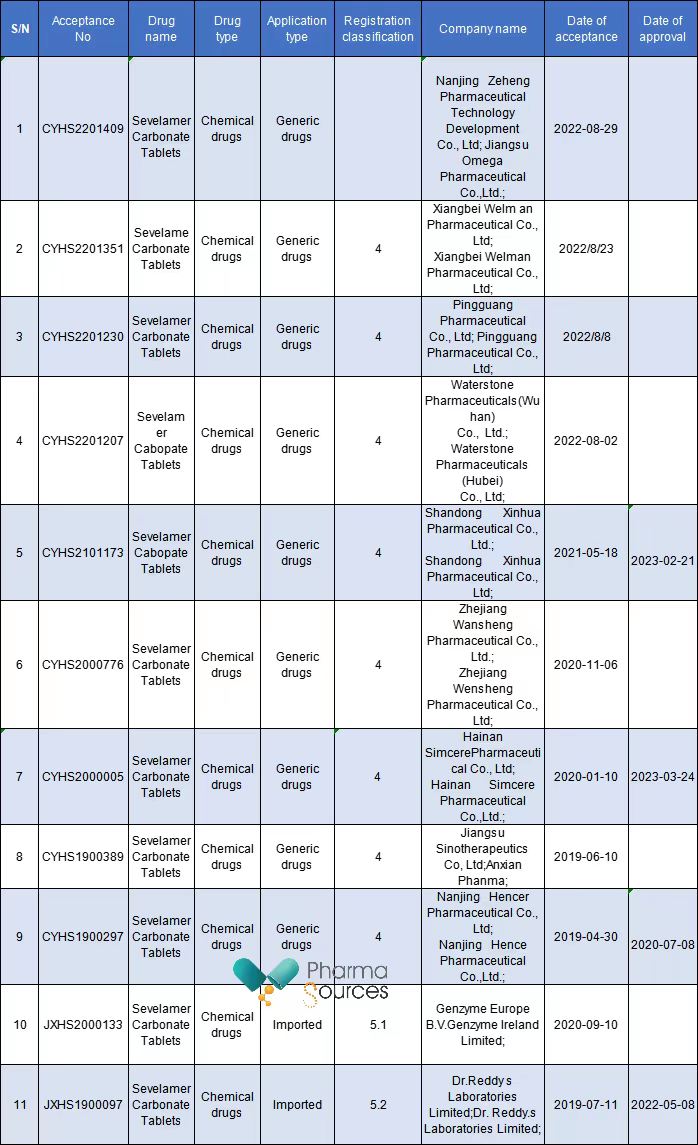
It can be said that Sevelamer Carbonate Tablets have entered the fierce competition stage in the Chinese market.
1. Zhang LX. Prevalence of chronic kidneydisease in China:a cross-sectional survey[J]. Lancet, 2012,379(9818):815-22.
2. High-end generic drug of sales volume of RMB 600 million! A Shandong pharmaceutical company was approved as the second Chinese company.
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


