Yefenghong/PharmaSourcesMarch 01, 2023
Tag: Bispecific , Biopharma , GB261
Currently, the R&D of CD20/CD3 bispecific antibody is still in the early stage. In China, few CD20/CD3 bispecific antibodies have entered the clinical stage, among which GB261 from Genor Biopharma has a good progress.
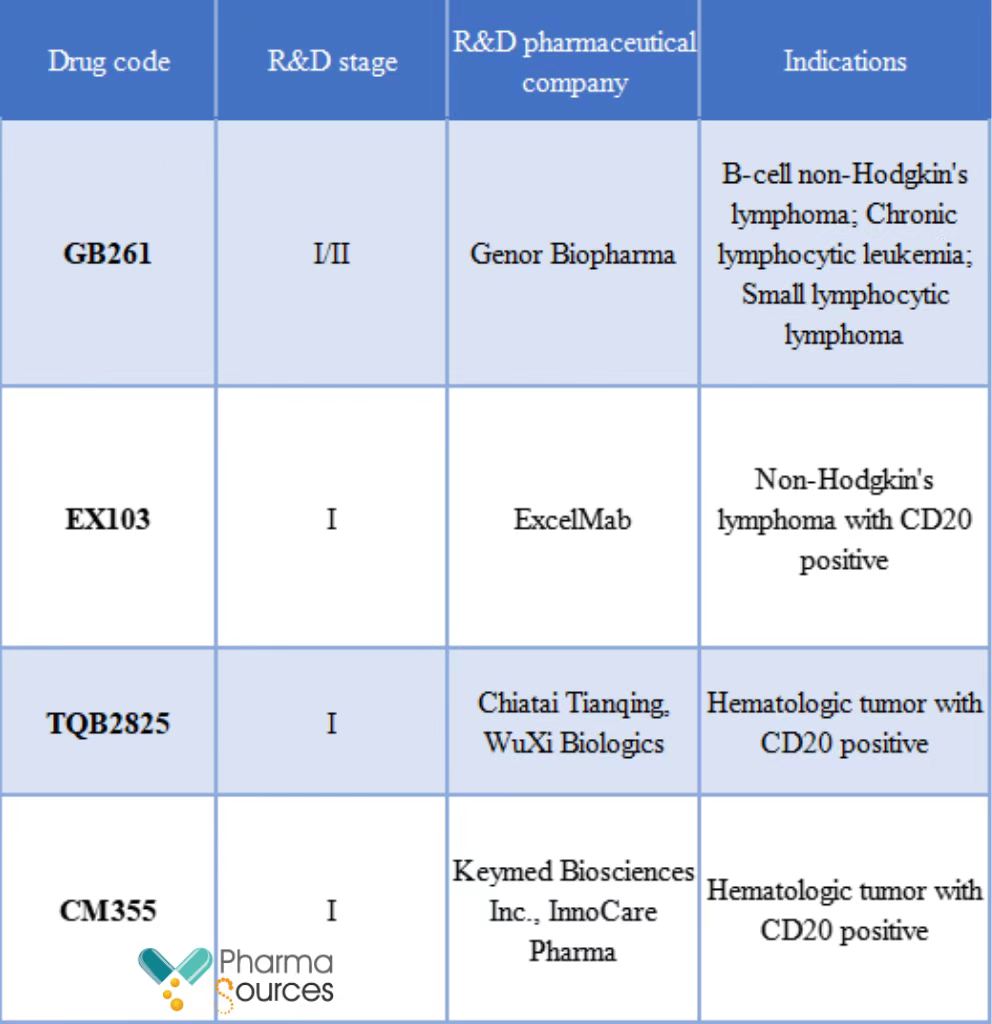
The R&D progress of some CD20/CD3 bispecific antibodies in China (public data)
Genor Biopharma's GB261 was designed by AB Studio through high-low bispecific antibody platform firstly, and then developed by Genor Biopharma for further. During the R&D of CD20/CD3 bispecific antibody, the affinity between CD3 antibody and antigen is the key. The reason is that if the affinity is too strong, T cell will be activated excessively, leading to CRS; if the affinity is too week, the therapeutic effect will be greatly reduced.
In the trial design, for GB261, common light chain was adopted, and ETYY (KIH reinforced form) technology was used to avoid mispairing between heavy chains. The bispecific antibody with high CD20 affinity and ultra-low CD3 affinity was obtained, by binding amino acids with antigen of CD3 monoclonal antibody in one side VH of rituximab, instead of binding amino acids with CD20, and adjusting antigen bonding force of CD3 appropriately. This bispecific antibody inhibited cytokine storm based on remaining the effective activation of T cell.
In vitro test, compared with target bispecific antibody being in the clinical stage, GB261 shows a good balance beween safety and efficacy, which the release of cytokine is much lower than the similar bispecific antibody in clinical stage, inhibiting the proliferation of cancer cells resistant to rituximab significantly. In addition, only focusing on tumor cells, GB261 weakens the affinity with CD3 and remains the effect of IgG1Fc.
ExcelMab's EX103 is developed by using ExMab bispecific antibody technology platform, which combines two different specific antibody molecules with protein engineering technology to obtain a stable drug entity with a low immunogenicity. By reforming Fc and Fab region, the platform makes two antibodies with different specificities compose heterodimers, resolving the mispairing between the light chain and heavy chain. EX103 has a better efficacy and safety compared with similar molecules in research abroad, which CRS release level is only one sixth of similar molecules. In 2021, EX103 obtained the drug clinical trial approval document, and now it is in the Phase I clinical stage.
Chiatai Tianqing and WuXi Biologics' TQB2825 was developed by WuXi Biologics using its WuXiBody bispecific antibody technology platform. Among them, CD3 antibody is from hybridoma and CD20 antibody sequence is from ofatumumab or rituximab. Clinical trial application of TQB2825 got the implied license from CDE in July 2021 for the treatment of hematologic tumor with CD20 positive.
Keymed Biosciences Inc. and InnoCare Pharma's CM355, developed by using nTCE bispecific antibody platform, can specifically bind CD20 positive target cells and CD3 positive T cells, gather the immune T cells around the target cells, activate T cells, and induce TDCC to kill target cells, which is used for treating the CD20 positive B cell hematologic tumor. CM355 obtained the clinical trial approval document in 2021 in China, and was administrated to the patient firstly in January 2022 in China, which is intended for the treatment of hematologic tumor with CD20 positive. Preclinical data indicate that CM355 has a stronger TDCC activity and controlled safety compared with other similar products.
In addition, Odronextamab, a CD20/CD3 bispecific antibody developed by American Regeneron using its Veloci-Bi bispecific antibody platform, has finished the Phase II clinical research at present. In China, Zai Lab invested US$190 million to introduce Odronextamab in 2020, obtaining its development rights in Greater China. In the clinical research named as ELM-1, odronextamab showed an encouraging activity in the DLBCL patients after at least second-line therapy. The results showed that for the DLBCL patients treated with a dose of ≥80mg odronextamab, ORR was 53% and CR rate was 53%. The sustained response rate was 88% at 12 months.
As an ideal tumor therapeutic target, CD20/CD3 attracts the layout of many pharmaceutical companies at home and abroad. R&D of CD20/CD3 bispecific antibody can not only receive a large amount of capital investment, but also meet the patients' need. Like the R&D of many innovative drugs, CD20/CD3 bispecific antibody's R&D will have a better development only based on clinical needs.
1. Bispecific antibodies: a mechanistic review of the pipeline. Nat Rev Drug Discov. 2019 Aug;18(8):585-608.
2. Won-Seog Kim, Tae Min Kim, Seok-Goo Cho, et al. Odronextamab in Patients with Relapsed/Refractory (R/R) Diffuse Large B-Cell Lymphoma (DLBCL): Results from a Prespecified Analysis of the Pivotal Phase II Study ELM-2. 2022 ASH. Abstract#444.
3. Cheah CY, Bartlett NL, Assouline S, et al; Mosunetuzumab Retreatment Is Effective And Well-Tolerated In Patients With Relapsed Or Refractory B-Cell Non-Hodgkin Lymphoma. EHA 2022 Poster presentation, P1124.
Morschhauser F, Bishton M, Eyre TA, et al. Mosunetuzumab in Combination with Lenalidomide Has a Manageable Safety Profile and Encouraging Activity in Patients with Relapsed/Refractory Follicular Lymphoma: Initial Results from a Phase Ib Study. ASH 2021 Oral 129.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


