Yi/PharmaSourcesMarch 01, 2023
Tag: Humira , autoimmune disease , biosimilar , AbbVie
On January 31, Amgen announced that its Humira (Adalimumab) biosimilar, Amjevita (adalimumab-atto), had entered into the American market officially, marking Humira's 20-year market exclusiveness has ended completely. And Amjevita is also the first adalimumab biosimilar approved by FDA.
Recently, the Category 5.1 application for registration of "Upadacitinib Sustained Release Tablet" delivered by AbbVie was accepted by CDE. Upadacitinib (Rinvoq), a kind oral selective JAK1 inhibitor developed by AbbVie, has had 3 approved indications in China at the moment: atopic dermatitis, rheumatoid arthritis and psoriatic arthritis. Noticeably, the three approved indications have entered the national medical insurance in 2023.
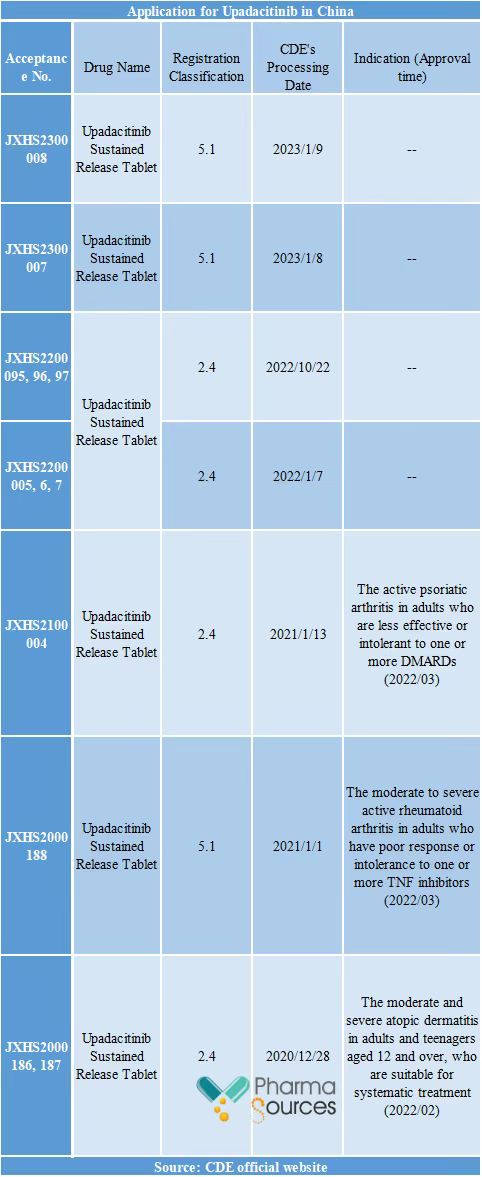
According to the drug clinical trial registration and information publicity platform of CDE, Upadacitinib (R&D code: ABT-494) has been successively approved for 6 indications in America, involving ulcerative colitis, Crohn's disease, axial spondyloarthritis, systemic lupus erythematosus and so on, and has registered 15 clinical trials in total in China. The author speculates that the Upadacitinib's indication declared this time is: axial spondyloarthritis.
In addition, Upadacitinib is developed to treat diseases including Crohn's disease (it has been declared for marketing), Takayasu arteritis (Phase III clinic), giant cell arteritis (Phase III clinic), juvenile idiopathic arthritis (Phase III clinic), systemic lupus erythematosus (Phase II clinic), hidradenitis suppurativa (Phase II clinic), etc.
Upadacitinib is the rising star of autoimmune disease pipeline in AbbVie, which sales volume reached US$1.651 billion in the third year of marketing, and is expected to break through US$2 billion by 2022. It is expected that the sales volume of Upadacitinib will reach a new high in the future with the new indications approved for marketing.
But while reviewing the marketing research of Pfizer's JAK inhibitor, Xeljanz, FDA found that the incidence of MACE and cancer in Xeljanz was higher than that in Humira and Enbrel, then the similar JAK inhibitors, Upadacitinib and Olumiant (Eli Lilly), were also questioned for the security, and a black box warning has been added to the label. Bernstein's analyst, Ronny Gal, reduced the peak expection of sales volume of Rinvoq from US$17.2 billion to US$11.2 billion.
Autoimmune disease is the business segment with the highest revenue in AbbVie, which also has Skyrizi approved in the European, American and Japanese markets, as well as many products in development. Humira, as the dominated product, has always been regarded as the king of medicine from 2012 to 2020. But Humira will drop from the top soon, for that its European drug patent expired in October 2018, and its American patent will aslo expire in 2023. On January 31, Amgen's adalimumab biological analogue, Amjevita (adalimumab-atto), had entered into the American market officially, which is the main market of Humira. Humira's sales volume will drop sharply along with the marketing of the biological analogues and their similar products. Evaluate Pharma predicted that global sales volume of Humira would drop to US$6.83 billion in 2026.
According to the data of Frost & Sullivan, the global drug market of autoimmune disease is predicted to increase from US$116.9 billion in 2019 to US$163.8 billion in 2030. New drugs will appear constantly in the field of autoimmune diseases, and we hope that biological innovative drugs can perform perfectly in the future.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


