Zhulikou/PharmaSourcesFebruary 15, 2023
Tag: first generic drugs , NMPA Approval , original drugs
At present, generic drugs occupy a larger share in the medical market in China. According to incomplete statistics, based on the drug approval date, 984 approval documents of generic drug made in China were approved by National Medical Products Administration in 2022 (Category 3/4/6 of generic drug, not containing the supplement application; among which Category 6 is the approved varieties declared according to the original drug registration classification), including 379 varieties, in which 71 first generic drugs were approved (See Table 1 for details. Please correct me if there are any mistakes or omissions in the statistics.)
Generic drug refers to the substitute, which has the same active ingredient, formulation, usage and dosage, administration route and therapeutic effect with the original drug, with the important economic and social benefits, such as reducing medical expenditure, improving the drug accessibility, and enhancing medical service level.
As early as 1984, FDA put forward the concept of first generic drug, meaning that the first generic drug applicant will have the market monopoly right for 180 days in the case that "the patent is invalid or the approval of the drug being applied for will not infringe the patent", according to "Drug Price Competition and Patent Restoration Act" (also known as " Hatch-Waxman Amendment"). No official document has yet defined "first generic drug" in China. Though the 2022 "Regulations for Implementation of the Drug Administration Law" (draft for comment) mentions the similar protection clause, but it has not been finalized yet. At present, the accepted statement mainly refers to that "first generic drug" is "the generic drug that China's drug enterprise firstly imitates and markets", which it can be a generic drug whose original drug has not been marketed in China (Class 3 of chemical drug), and it can also be a generic drug whose original drug has been marketed in China (including the import) (Class 4 of chemical drug).
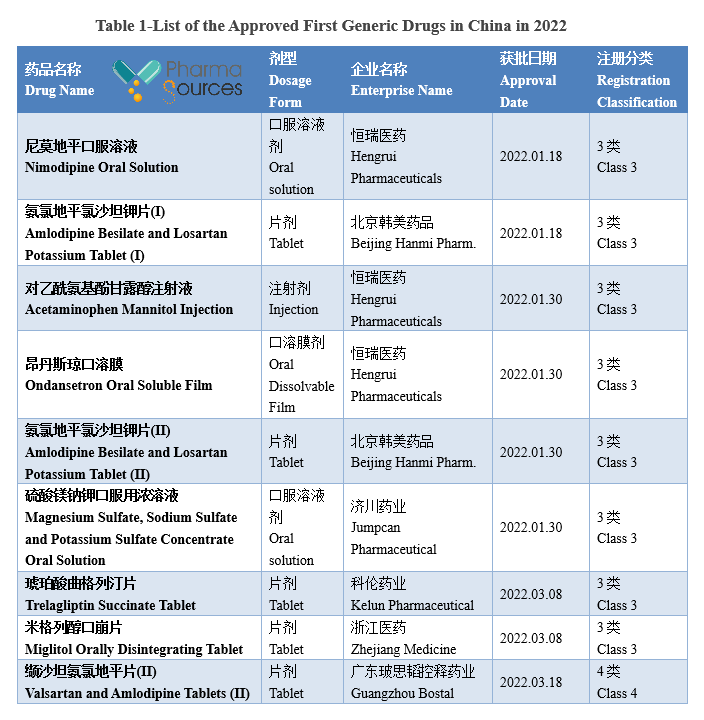
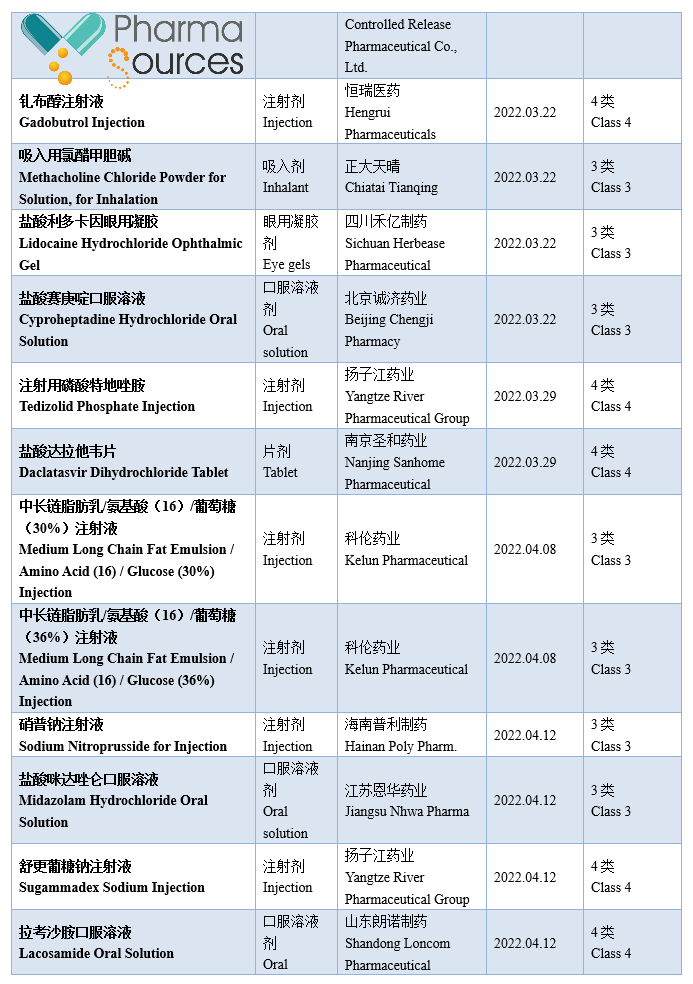
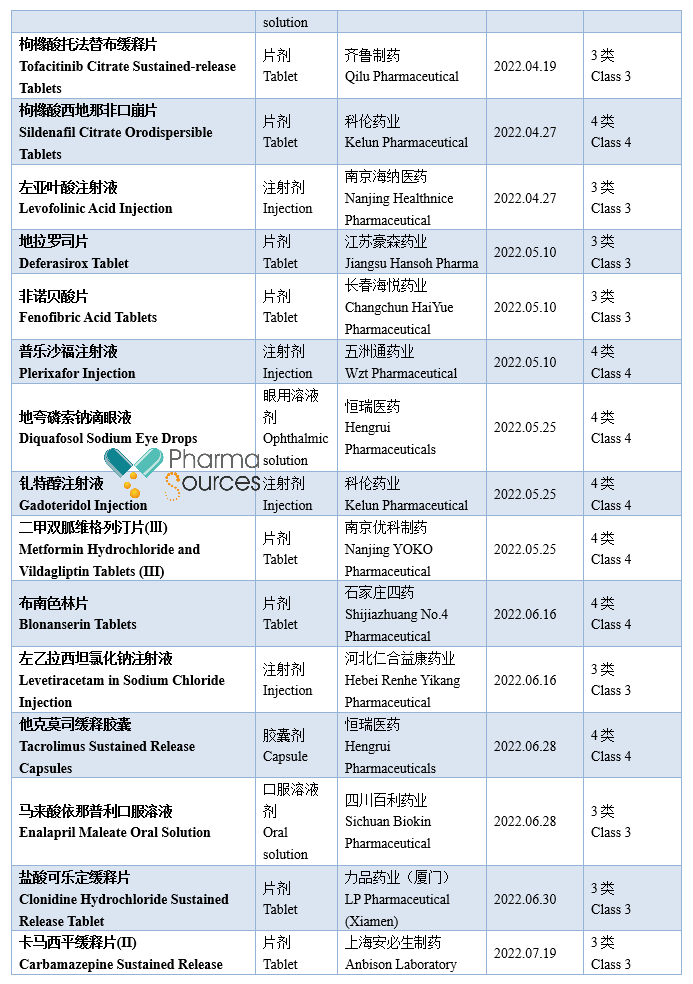
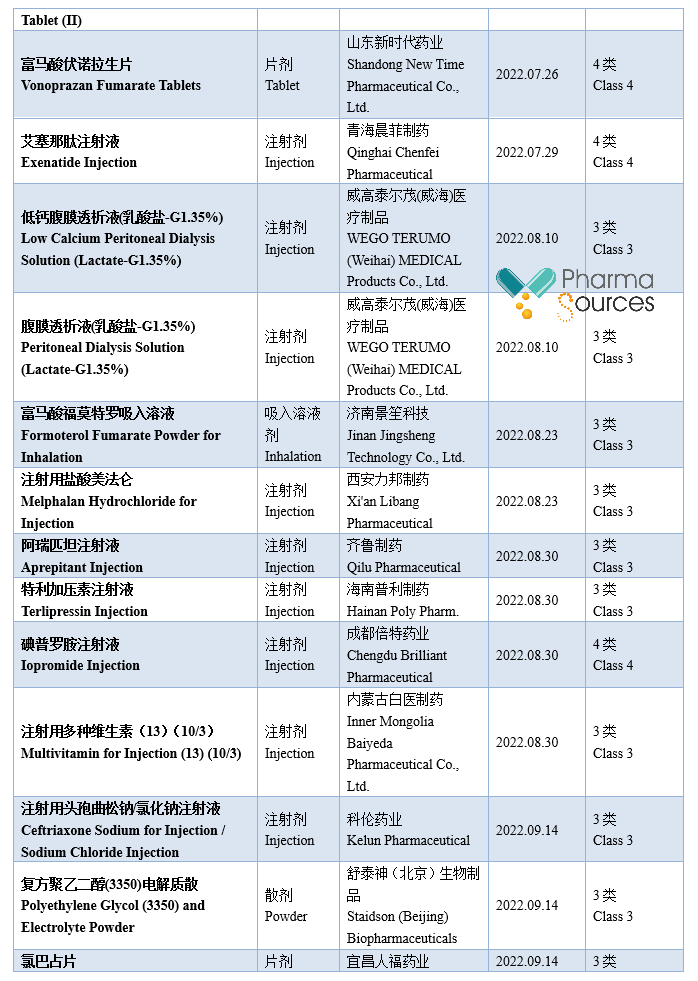
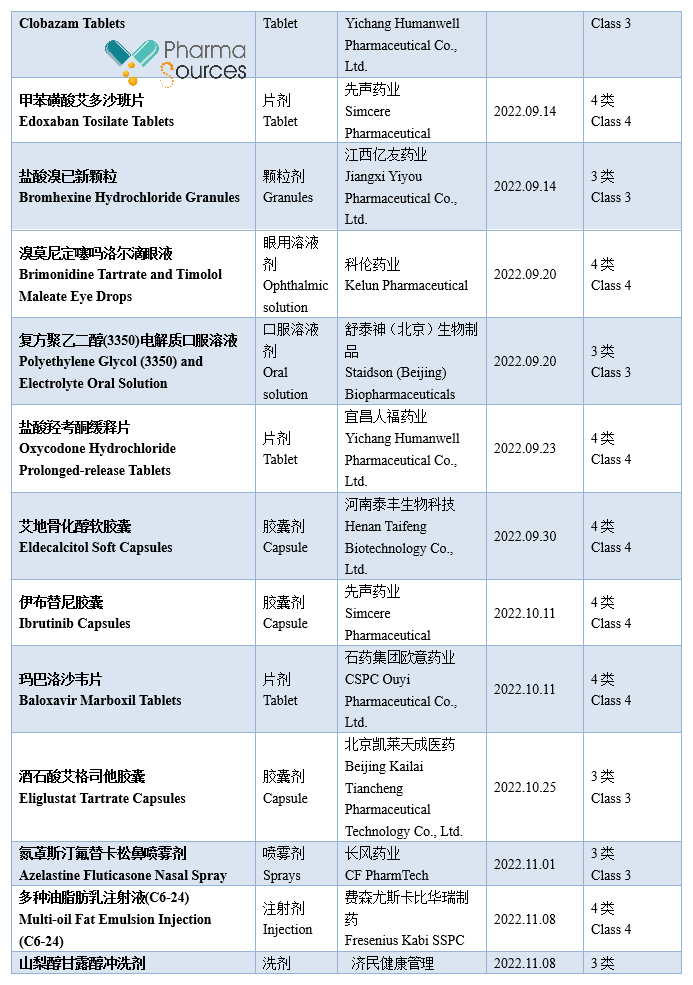
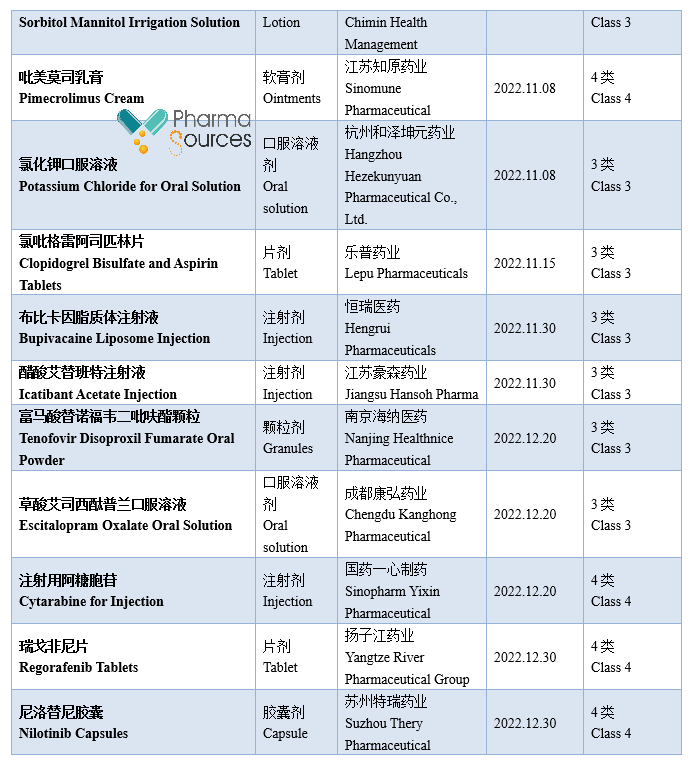
Note: This article doesn't provide any judgement on the value and suggestion on the investment.
1- NMPA Database
2- DXY Insight Database
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


