PharmaSources.com/YiMay 23, 2022
Tag: TAA06 , B7-H3 , Omburtamab
On April 11, CDE’s official website revealed that the application for an Investigational New Drug, the new first-in-class [TAA06 Injection] by PersonGen BioTherapeutics (Suzhou) Co., Ltd. was accepted. Based on public information, TAA06 is an independently developed B7-H3-targeted CAR-T therapy of PersonGen. The drug was granted the Orphan Drug Designation (ODD) for treating neuroblastoma by the FDA (Food and Drug Administration) in March of this year.
![The application of the new first-in-class [TAA06 Injection] by PersonGen BioTherapeutics (Suzhou) Co., Ltd. was accepted The application of the new first-in-class [TAA06 Injection] by PersonGen BioTherapeutics (Suzhou) Co., Ltd. was accepted](https://eimg.pharmasources.com/upload/image/20220523/R3StUWVJDxC2CPmtMpSXV6BWw0hid3HGpx13uXnF.png)
B7-H3 (CD276) is a type I transmembrane protein and a member of the B7 immune co-stimulation and co-suppression family. It is overexpressed in solid tumors such as bladder cancer, prostate cancer, and melanoma, but its expression is limited in normal tissues. The early discovery of B7-H3 was mainly demonstrated to function as a co-stimulatory receptor, promoting the proliferation of CD4+ and CD8+ T cells, inducing cytotoxic T cells, and producing immunostimulatory functions by selectively stimulating interferon γ (IFN-γ) in the context of T cell receptor signaling. As the research progressed, there is increasing evidence showing that B7-H3 plays a major role in immune cells as a co-inhibitor, facilitating the evasion of tumor cells from immune surveillance. Therefore, the overexpression of B7-H3 is related to the poor prognosis of tumor patients and the invasion and metastatic potential of tumor in vitro models.
B7-H3 is an emerging target for immunotherapy. Among all the products, omburtamab by Y-mAbs Therapeutics progresses the fastest and has resubmitted the BLA (Biologics License Application) for the treatment of pediatric patients with the central nervous system (CNS) or leptomeningeal metastasis neuroblastoma to the FDA in early April. Omburtamab is a radionuclide iodine-131-labeled B7-H3-targeted monoclonal antibody that targets B7-H3-expressing cells in solid tumors and binds to the FG loop-dependent conformation, a critical region of biological function in the B7-H3 molecule. In December 2020, SciClone Pharmaceuticals reached an agreement with Y-mAbs Therapeutics and obtained exclusive rights to the co-development, registration, and commercialization of the drug and GD2 targeted monoclonal antibody-Danyelza (naxitamab-gqgk) in Greater China (including Chinese mainland and Hong Kong/Macau/Taiwan Regions of China). Moreover, Y-mAbs Therapeutics has developed 177Lu-omburtamab-DTPA radiolabeled by lutetium-177 for the treatment of pediatric patients with relapsed or refractory medulloblastoma and adult patients with positive CNS tumors or leptomeningeal metastasis, among which the medulloblastoma indication was granted rare pediatric disease designation (RPDD) by the FDA.
And the B7-H3-targeted monoclonal antibody enoblituzumab developed by MacroGenics also sees good progress, now in Phase II clinical trials. Enoblituzumab is an immune optimized anti-B7-H3 monoclonal antibody. It combines the exclusive Fc optimization technology platform of MacroGenics, with unique antibody advantages and therapeutic potential. The published interim analysis results of Phase I clinical trials of enoblituzumab for refractory solid tumors show that: the drug can be tolerated up to 15 mg/kg without maximum tolerated dose (MTD) and dose-limiting toxicity (DLT). In July 2019, I-Mab Biopharma reached an agreement with MacroGenics to obtain the exclusive development and commercialization rights of the drug in Greater China (including the Chinese mainland, Hong Kong/Macau/Taiwan Regions of China).
In addition to monoclonal antibodies, pharmaceutical companies have also developed bispecific antibodies, antibody-drug conjugates (ADC), and CAR-T therapies around B7-H3 targets, as shown in the table below.
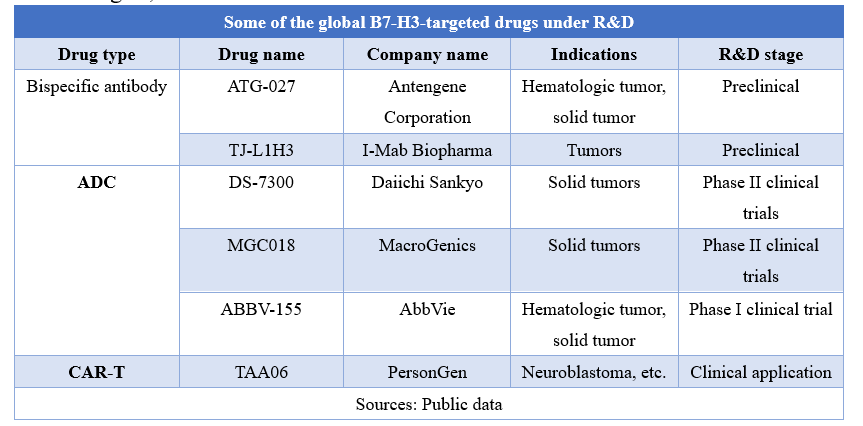
Ø ATG-027 is a B7H3 and PD-L1-targeted bispecific antibody researched and developed by Antengene Corporation, which is developed for the treatment of hematological malignant tumors and solid tumors.
Ø DS-7300 is an ADC developed by Daiichi Sankyo with its proprietary DXd technology. It consists of a humanized anti-B7-H3 monoclonal antibody and a new topoisomerase I inhibitor coupled with a tetrapeptide linker. Preclinical research reveals that the drug showed activity in the expression of B7-H3 tumors, and the activity was related to the target expression level. Phase I/II clinical trials data presented at the 2021 European Society of Medical Oncology (ESMO) Annual Meeting showed that in clinical trials of a series of patients with solid tumors (n=70), including Metastatic Castration-Resistant Prostate Cancer, head and neck squamous cell carcinoma, small cell lung cancer, endometrial cancer, esophageal squamous cell carcinoma and squamous NSCLC, 15 patients achieved partial response (PR), of which 10 had confirmed PR and 5 had PR pending confirmation.
Ø MGC018 is a B7-H3-targeted ADC that delivers the DNA alkylating agent duocarmycin to tumor cells expressing B7-H3. Duocarmycin is capable of damaging the DNA of dividing and nondividing cells, leading to cell death. Results from the Phase l dose-escalation clinical trial presented at the 2021 ASCO Annual Meeting showed that the drug demonstrated preliminary antitumor activity with excellent efficacy, particularly in patients with advanced metastatic castration-resistant prostate cancer.
Ø ABBV-155 (mirzotamab clezutoclax) is a B7-H3-targeted ADC with a load of BCL-XL inhibitor which can promote apoptosis.
Overall, the competition for B7-H3-targeted drugs is not that fierce currently, and the types of drugs under R&D are relatively diversified, most of which are in the initial stage with the constant exploration of indications. Chinese pharmaceutical companies are also actively exploring the field of B7-H3-targeted drugs, in addition to independent R&D, but also from foreign pharmaceutical companies to introduce. Omburtamab, the world's first B7-H3-targeted drug, is expected to debut as soon as possible, to drive the R&D of competitive pharmaceutical companies to great extent.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


