Neeta RatanghayraMay 20, 2022
Tag: ADC , Gemtuzumab ozogamicin , brentuximab vedotin , polatuzumab vedotin
Antibody-drug conjugates (ADCs) have made significant progress in tumor therapy and show a promising future. As per insights from Clarivate experts, the global market for currently marketed ADCs is expected to cross US$16.4 billion by 2026. However, designing an ADC is a challenge requiring a careful combination of the antibody, linker, and the cytotoxic drug.
The approval of Pfizer’s Mylotarg® in 2000 marked the beginning of an era of targeted oncology therapy. But it took almost a decade for the second ADC, Brentuximab vedotin (Adcetris®), to enter the market. What were the problems with the earlier ADCs?
This article highlights the recent advances in ADCs and lessons learned from the developmental challenges faced with the earlier ADCs.
Antibody-drug conjugates are biotherapeutics that utilize antibodies to selectively deliver cytotoxic (payloads) drugs to the tumor site.
The antibody in an ADC is attached to the cytotoxic drugs via a linker system. The linkers act as a specific bridge, helping the antibody release the cytotoxic drug selectively and accurately at tumor sites. The linkers also help maintain the ADCs’ stability during their preparation, storage, and systemic circulation.
The antibody in the ADC is designed to target a specific antigen (receptor) that is highly expressed in tumor cells. By selectively delivering the cytotoxic drug to tumors, ADCs limits their systemic exposure, leading to greater efficacy and minimal side effects.
In 2000, the U.S. Food and Drug Administration (FDA) approved Pfizer’s Mylotarg® (gemtuzumab ozogamicin) for acute myeloid leukemia (AML). In 2010, Pfizer voluntarily withdrew Mylotarg in the U.S. after a confirmatory trial failed to demonstrate clinical benefit and showed increased toxicity compared to chemotherapy. However, in September 2017, the FDA reapproved Mylotarg for adults with newly diagnosed CD33-positive AML and relapsed or refractory CD33-positive AML in patients aged 2 years and older. The FDA specifically highlighted the modified dosing regimen that improved gemtuzumab ozogamicin’s safety and efficacy and led to its reapproval.
It Took Almost a Decade for The Second ADC To Enter the Market - What were the problems with the earlier ADCs?
Seagen’s Brentuximab vedotin (Adcetris®) was the second ADC to be approved. The drug received FDA approval in 2011. A gap of 11 years for the second ADC to enter the market highlights the challenges which manufacturers faced while developing the earlier ADCs.
BR96–doxorubicin and KS1/4–methotrexate were the first ADCs to be developed. These ADCs were basically chemotherapy drugs linked to murine antibodies using a non-cleavable linker. Both drugs were unable to show clinical benefit, and their further development was discontinued.
Next, the researchers experimented with combinations of potent cytotoxic agents and humanized monoclonal antibodies. Gemtuzumab ozogamicin was a humanized anti-CD33 IgG4 antibody, conjugated to calicheamicin using an acid-labile hydrazone linker. Gemtuzumab ozogamicin showed increased efficacy in clinical studies and became the first ADC to enter the market in 2000. However, in subsequent studies, the drug showed poor efficacy and increased toxicities which lead to its withdrawal in 2010. (Gemtuzumab ozogamicin was reapproved in 2017 by the FDA).
Unstable linker, high amount of unconjugated antibody, poor CMC (chemistry, manufacturing, and control) properties, and high toxicity were some of the disadvantages of the first-generation ADCs. Learning from these drawbacks lead to the development of the second-generation ADCs.
The second-generation ADCs showed better safety, efficacy, and CMC characteristics. These ADCs were introduced after optimization of monoclonal antibodies isotypes, cytotoxic payloads, and the linkers. Several potent chemotherapy drugs, such as auristatins and mytansinoids, with improved water solubility and coupling efficiency, were also discovered. The improved linkers helped achieve better plasma stability and homogeneous drug-to-antibody ratio (DAR) distribution.
Though better than the first-generation ADCs, there were still challenges with the second-generation ADCs. Off-target toxicity led to insufficient therapeutic windows. Problems of aggregation or rapid clearance (hence decreased efficacy) were observed in ADCs with high DAR. Competition with unconjugated antibodies for antigen binding was another issue.
Optimization of DAR using site-specific conjugation played a pivotal role in developing successful third-generation ADCs. Site-specific conjugation led to improved pharmacokinetics, desired cytotoxicity, and homogeneous ADCs with DAR 2 or 4. Consistent DARs also led to less off-target toxicity.
The use of fully humanized antibodies instead of chimeric antibodies in the third generation ADCs helped reduce immunogenicity. Research is ongoing to replace intact mAbs with antigen-binding fragments (Fabs), which are more stable in systemic circulation and may be internalized more readily by tumor cells.
Another feature of the third-generation ADCs is the use of potent payloads such as pyrrolobenzodiazepine dimer (PBD), tubulysin, and immunomodulator. Fleximer platform and hydrophilic linker modulation such as PEGylation are some novel developments in the linker space.
The latest innovations and developments confer third-generation ADCs with higher stability and efficacy as well as lower toxicity.
Antibody–Drug Conjugates Currently on The Market
The crafting of a perfect ADC is easier said than done. But despite the challenges, many companies have succeeded in designing the right combination. As of December 2021, there are 14 ADC drugs approved worldwide and over 100 candidates are at various stages of clinical trials. Here is a quick overview of the approved ADCs.
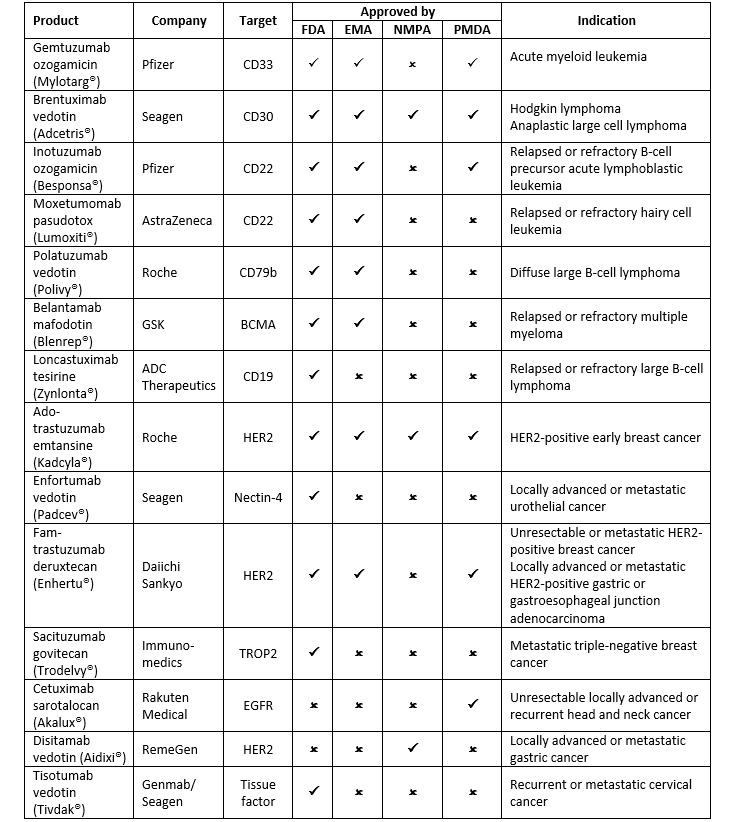
The success of recent drugs has ignited new hopes in the field of ADCs. Learnings from past failures and advances in chemistry have encouraged several companies to invest in this space. Below is a list of selected candidates in the late phase of development:
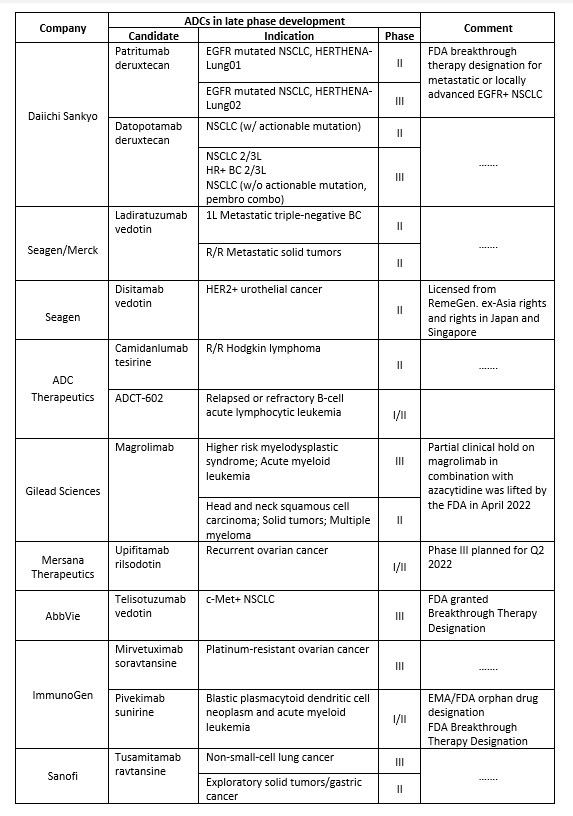
*R/R, relapsed or refractory; NSCLC, Non-small cell lung cancer;
EGFR, epidermal growth factor receptor;
FDA, Food and Drug Administration; EMA, European Medicines Agency;
HER, human epidermal growth factor receptor; BC, Breast Cancer.
The ADC market is set to witness phenomenal growth with several candidates across various cancer types entering late-phase development. Compared to the earlier generation, new generation ADCs come with enhanced specificity and cytotoxicity profiles, but challenges exist. Inadequate tumor targeting, complex pharmacokinetics, and issues with payload release and drug resistance can be tough to handle. The use of advanced analytical techniques, innovations in the main components (antibody, payload, and linker), and novel bioconjugation platforms can help shape the future development of ADCs.
1. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther. 2022;7(1):93.
2. do Pazo C, Nawaz K, Webster RM. The oncology market for antibody-drug conjugates. Nat Rev Drug Discov. 2021;20(8):583-584.
3. Dean AQ, Luo S, Twomey JD, Zhang B. Targeting cancer with antibody-drug conjugates: Promises and challenges [published correction appears in MAbs. 2021 Jan-Dec;13(1):1966993]. MAbs. 2021;13(1):1951427.
4. Tong JTW, Harris PWR, Brimble MA, Kavianinia I. An Insight into FDA Approved Antibody-Drug Conjugates for Cancer Therapy. Molecules. 2021;26(19):5847.
5. Selby C, Yacko LR, Glode AE. Gemtuzumab Ozogamicin: Back Again. J Adv Pract Oncol. 2019;10(1):68-82.
Neeta Ratanghayra
Freelance Medical Writer
Neeta Ratanghayra is a freelance medical writer, who creates quality medical content for Pharma and healthcare industries. A Master’s degree in Pharmacy and a strong passion for writing made her venture into the world of medical writing. She believes that effective content forms the media through which innovations and developments in pharma/healthcare can be communicated to the world.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


