David Orchard-WebbMay 12, 2022
Tag: Oral Vaccine , Dragee-candy , COVID-19
A vaccine is a biologic that improves immunity to disease. Vaccination is considered the most effective means of controlling infectious disease-related morbidity and mortality. Vaccination prevents over 2.5 million child deaths each year, globally, according to the World Health Organization (WHO).1
Half of the top ten leading causes of death in low-income countries are caused by infectious diseases including lower respiratory infections (e.g. COVID-19 and other causes of pneumonia), human immunodeficiency virus (HIV), diarrheal disease, malaria, and tuberculosis.2 Some prevalent pathogens including HIV lack an effective vaccine, however, an estimated 20% of prevalent pathogen-induced deaths result from vaccine-preventable diseases. Furthermore, this problem is not limited to low-income countries, COVID-19 is a top-three cause of death in the United States, despite the availability of vaccines.3 This indicates an unmet medical need to improve vaccine compliance rates and accessibility.
Vaccines are typically delivered via subcutaneous (SC) or intramuscular (IM) injection. Dendritic cells (DCs), however, can be found in higher concentrations in other areas of the body, such as the upper layers of the skin (epidermis and dermis), nasal mucosa and the gastrointestinal (GI) tract. The oral formulation of vaccines is therefore an ideal solution for delivery to the GI tract.
The majority of vaccinations are administered to children. Oral vaccines potentially have several advantages over conventional subcutaneous or intramuscularly injected vaccines. Firstly, compliance with oral vaccination is much more straightforward than conventional vaccines, especially for children, requires less equipment (syringes, needles), does not require a health professional to administer, and oral vaccine distribution is simpler. Secondly, oral vaccination may be more effective, offering both mucosal immunity and cellular immunity in addition to conventional systemic antibody-mediated immunity.2
Oral vaccine technology does face several challenges posed by the digestive tract such as pH changes and digestive enzymes that can degrade the vaccine components. For an oral vaccine to be successful the following conditions must be met:
1) successful delivery of active antigen (or encoding DNA) to intestinal cells
2) antigen transport across the mucosal barrier
3) activation of antigen-presenting cells
Oral vaccines face one challenge, in particular, that is crucial to overcome. If the dose is not correct or the immune system is not sufficiently stimulated they can promote immune tolerance of the antigen, instead of the desired protective effect. The correct selection of immune-stimulating adjuvants is therefore crucial for the success of oral vaccines.4
Oral vaccines represent around a quarter, US $14.91 billion, of the total vaccine market, worth US $61.04 billion in 2021 (Figure 1). The North American vaccine market alone is reportedly worth US $29.43 billion. Vaccines delivered by syringe, microneedle and nasal spray represent a share of around US $44.6922 billion, US $1.3220 billion, and US $0.1158 billion respectively. Between 2022 to 2027 the oral vaccine market is expected to grow at a CAGR of 7.3%. The overall vaccine market is expected to grow at a similar rate of 10.20% between 2021-2028.
The oral vaccine market is currently dominated by formulations that target rotavirus, cholera, polio, and typhoid fever. All of these diseases initiate infections via the GI tract. Oral formulations of COVID-19 and Flu vaccines are also under development.
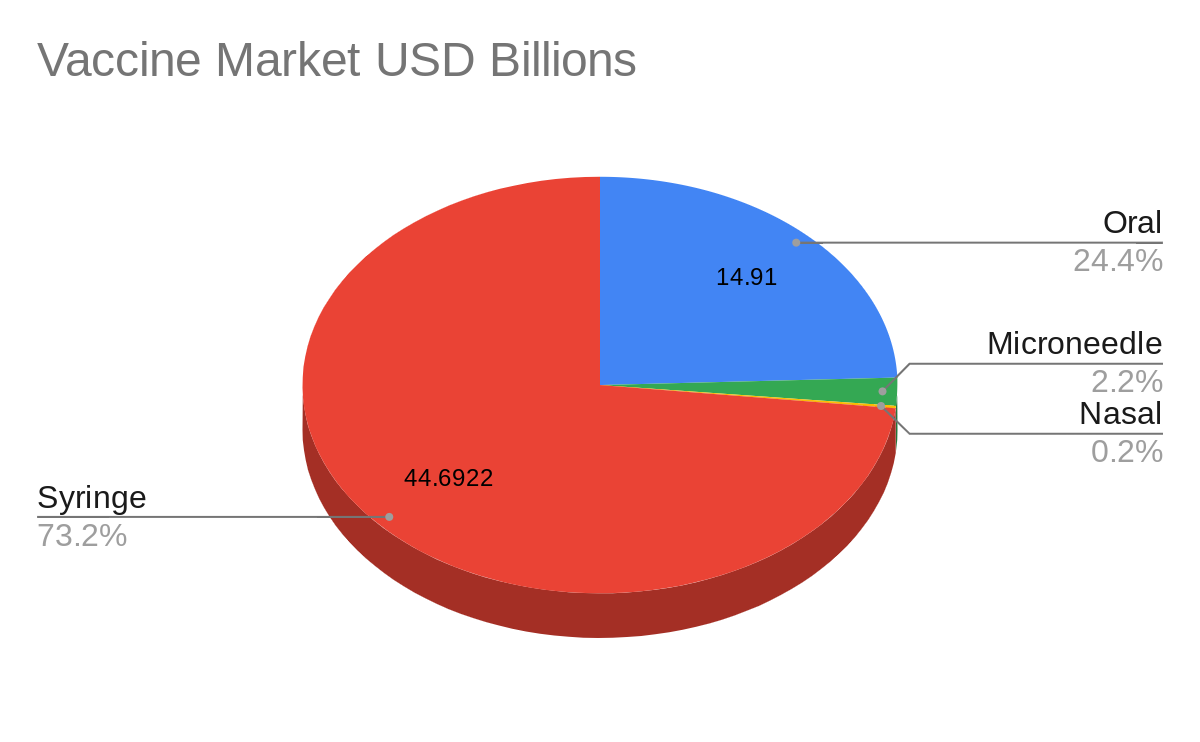
Figure 1: Vaccine market share in billion USD segmented by mode of delivery.
Major players in the oral vaccine market include Merck, GSK, Sanofi, Valneva, Lanzhou Institute of Biological Products Co. Ltd., Serum Institute Of India Pvt. Ltd., Unilab, Vabiotech, Bibcol, Emergent BioSolutions, TiantanBio, EuBiologics (South Korea), Bio-Med, and Halfkin Bio-Pharmaceuticals.
Merck’s RotaTeq is a live pentavalent oral vaccine that contains five reassortant rotaviruses. Human and bovine hosts were used to isolate the rotavirus parent strains of the reassortants. Four of the reassortant rotaviruses express one of the outer capsid proteins (G1, G2, G3, or G4) from the human rotavirus parent strain and the attachment protein (type P7) from the bovine rotavirus parent strain. A fifth reassortant virus expresses the attachment protein, type P1A[8], from a human rotavirus parent strain and the outer capsid protein of type G6 from a bovine rotavirus parent strain.
GSK’s ROTARIX contains a live, attenuated human rotavirus that replicates in the small intestine to produce immunity. The ROTARIX vaccine is derived from the human 89-12 strain, which belongs to the G1 serotype, the most prevalent strain worldwide, and P[8] genotype. Genotype P[8] is shared by most circulating strains, including serotypes G3, G4, and G9. Interestingly, the immunologic mechanism by which ROTARIX protects against rotavirus gastroenteritis is not fully understood. In addition, a relationship between antibody responses to rotavirus vaccination and protection against rotavirus gastroenteritis has not been established.
The Serum Institute Of India Pvt. Ltd.’s ROTASIIL-Liquid is indicated as a 3-dose series for active immunization of healthy infants from the age of 6 weeks for the prevention of gastroenteritis due to rotavirus infection.
Lanzhou Institute of Biological Products Co. Ltd. also produces oral rotavirus vaccines including a novel human-lamb reassortant rotavirus vaccine.
EuBiologics’ Euvichol-Plus® is an oral vaccine for the prevention of cholera, which predominates in Africa and Asia. Euvichol-Plus® has been supplied to cholera endemic or low and middle-income countries that regularly experience cholera outbreaks through the WHO Stockpile funded by Global Alliance for Vaccines and Immunizations (GAVI) following WHO PQ certification.
The cholera vaccine from Sanofi’s Indian subsidiary Shantha Biotechnics can be kept at ambient temperatures in excess of 40°C for up to two weeks immediately before administration, which could improve access to the vaccine in Asia and Africa, where it is used most. Sanofi India also produces an oral cholera vaccine called Shanchol. The use of these cholera vaccines in a controlled temperature chain (CTC) was endorsed by the World Health Organization (WHO).
Valneva’s DUKORAL® is an oral vaccine indicated for the prevention of diarrhea caused by Vibrio cholera and/or heat-labile, toxin-producing Enterotoxigenic Escherichia coli (ETEC), the leading cause of travelers’ diarrhea. It is authorized for use in the EU and Australia to protect against cholera and in Canada, Switzerland, New Zealand and Thailand to protect against cholera and ETEC. DUKORAL® is indicated for adults and children from 2 years of age who will be visiting cholera endemic areas and was prequalified by the WHO.
Unilab’s OraVacs™ inactivated whole-cell Vibrio cholerae recombinant cholera B subunit oral vaccine is an enteric-coated capsule that contains a light yellow or a light brown lyophilized powder. It consists of inactivated whole V. cholerae 01 cells combined with a recombinant B subunit of cholera toxin (rBS-WC).
Bio-Med’s Oral Polio Vaccine is prepared with attenuated Sabin Strain Polio viruses. The vaccine solution is a clear light pink color when in liquid form and turns pale yellow when frozen. Bio-Med’s Oral Polio Vaccine conforms to the Indian Pharmacopoeia.
Bibcol’s live oral polio vaccine (OPV) is bivalent containing suspensions of types 1 and 3 attenuated poliomyelitis virus (Sabin strain) prepared in primary monkey kidney cell culture. The vaccine is stabilized with 1M Magnesium chloride and contains the antibiotic Kanamycin at a concentration of 15 grams per dose.
Halfkin produces an oral bivalent type 1 & 3 oral poliomyelitis vaccine.
TiantanBio’s Trivalent Oral Poliovirus Vaccine in Dragee-candy is a prophylactic formulation for active immunization against poliomyelitis. It is prepared with attenuated poliovirus sabin strain by using human diploid cell culture that contains no extraneous agents.
Emergent BioSolutions’ Live Oral Ty21a is a live attenuated typhoid fever vaccine for oral dosing. It is the only oral vaccine indicated for use against Salmonella Typhi.
At the end of 2021 sales of Merck’s RotaTeq reached US $0.807 billion. In comparison, GSK’s sales of ROTARIX were US $0.732 billion during the same time period.
According to the company, EuBiologics is the largest supplier of an oral cholera vaccine in the world. Euvichol represents more than 80% of the cholera market segment. The company will be able to produce up to 50 million doses by 2023 following an expansion plan.
In the first half of 2021, revenues from Valneva’s cholera vaccine DUKORAL® sales reached €0.4 million compared to €12.1 million in the first half of 2020. Decreased travel due to the COVID-19 pandemic reduced demand for DUKORAL® during the course of the year.
Sales data for other products were not available.
REFERENCES
1. Donnelly, Ryan F. “Vaccine Delivery Systems.” Human Vaccines & Immunotherapeutics 13, no. 1 (January 26, 2017): 17–18. https://doi.org/10.1080/21645515.2016.1259043.
2. Vela Ramirez, Julia E., Lindsey A. Sharpe, and Nicholas A. Peppas. “Current State and Challenges in Developing Oral Vaccines.” Advanced Drug Delivery Reviews 114 (May 15, 2017): 116–31. https://doi.org/10.1016/j.addr.2017.04.008.
3. Ortaliza, Jared, Krutika Amin, and Cynthia Cox. “COVID-19 Leading Cause of Death Ranking.” Peterson-KFF Health System Tracker, March 24, 2022. https://www.healthsystemtracker.org/brief/covid-19-leading-cause-of-death-ranking/.
4. Wilson-Welder, Jennifer H., Maria P. Torres, Matt J. Kipper, Surya K. Mallapragada, Michael J. Wannemuehler, and Balaji Narasimhan. “Vaccine Adjuvants: Current Challenges and Future Approaches.” Journal of Pharmaceutical Sciences 98, no. 4 (April 2009): 1278–1316. https://doi.org/10.1002/jps.21523.
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


