YiApril 11, 2022
Tag: ICIs , LAG-3 inhibitor , relatlimab , PD-1
Lately, Opdualag, a fixed-dose compound preparation of BMS consisting of LAG-3 inhibitor relatlimab and PD-1 monoclonal antibody nivolumab, was approved by FDA for the treatment of adults and pediatric patients aged 12 years and older with unresectable or metastatic melanoma.
LAG-3 (lymphocyte activation gene-3), also known as CD233, is an immune checkpoint protein expressed on the surface of effector T cells and regulatory T cells to regulate the response, activation and growth of T cells. According to preclinical research, the inhibition of LAG-3 pathway can restore the effector function in T cell exhaustion and may promote the anti-tumor effect. In addition, the expression level of LAG-3 and LAG-3+ cell infiltration were found to correlate with the advanced tumor and the poor prognosis. The approval of Opdualag represents the relatlimab has become the 3rd immune checkpoint inhibitor (ICI) approved globally after CTLA-4 and PD-1/PD-L1.
Immune checkpoint inhibitors (ICI), which utilize the human's immune system to kill tumor cells by inducing and enhancing the immune response, is the most important achievement in the oncology field in the past decade. According to incomplete statistics, global regulatory agencies have already approved several antibody drugs for three immunosuppressant targets of PD-1, PD-L1 and CTLA-4 (see the table below for details), among which there are 11 PD-1 antibodies, 5 PD-L1 antibodies and 1 CTLA-4. It is remarkable that many Chinese pharmaceutical companies and healthcare supply companies also occupy a pivotal position in the market for the PD-1/L1 monoclonal antibody, with several products having been successfully approved for marketing. Furthermore, the global marketing of some products such as Sintilimab and Toripalimab have also been launched and BLAs have been submitted in the US.
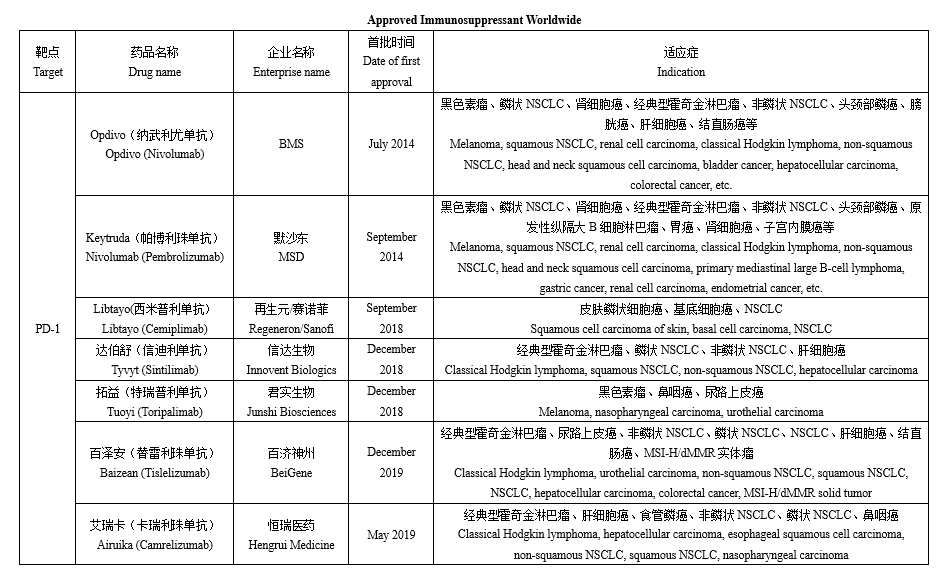
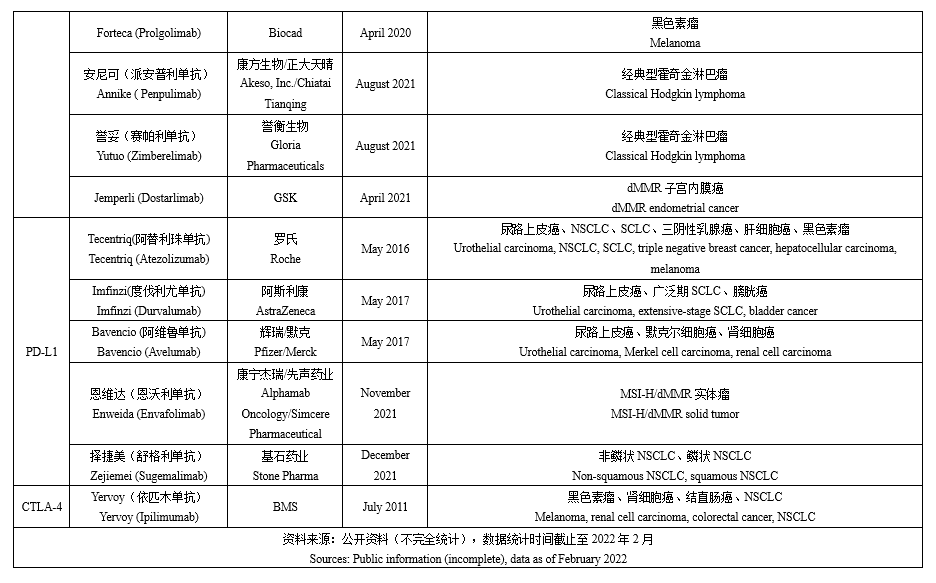
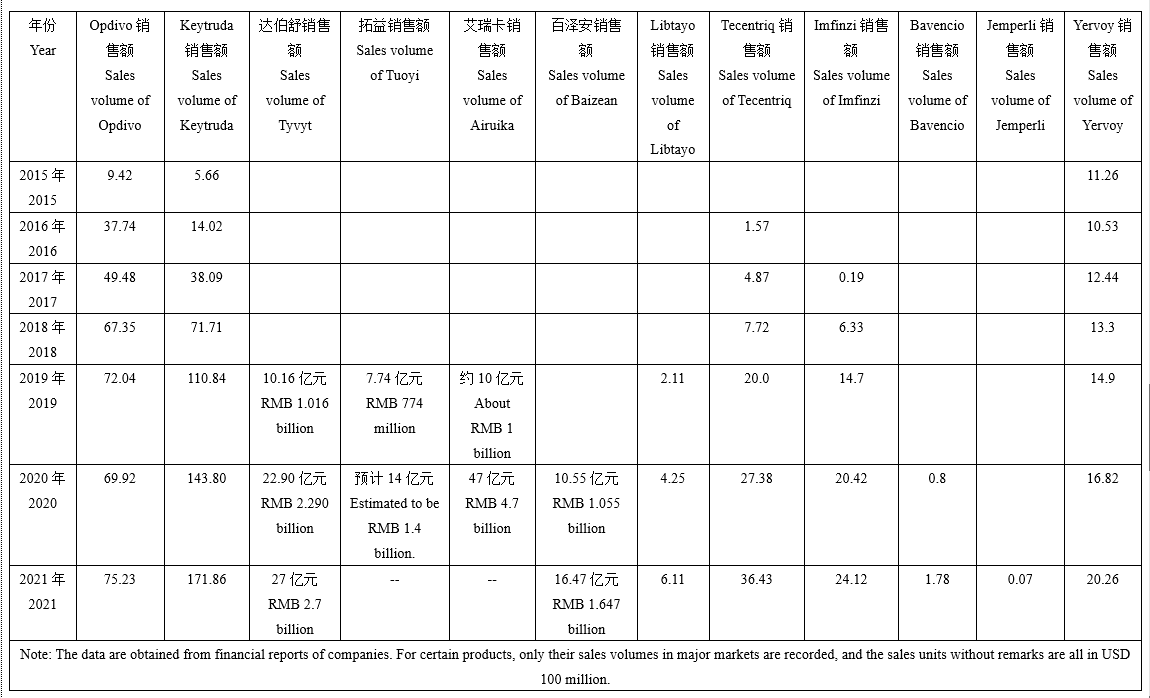
In recent years, ICIs have been growing at a rapid pace, with increasing approved numbers and indications, and a consequent expansion of their market size. Among them, Keytruda, a PD-1 monoclonal antibody, has the most approved indications and the highest sales, with MSD alone recording a sales volume of USD 17.186 billion. In addition, the sales volume of Keytruda has held the second position in the global drug sales ranking for two consecutive years (except for COVID-19-related products). The sales of ICIs in recent years show that the global ICIs market is expanding in volume, reaching at least USD 33.586 billion in 2021, and is expected to continue to expand in the future.
Sales of ICIs on Market in Recent Rears
In addition to the above inhibitory immune checkpoint targets, TIM-3, TIGIT, 4-1BB, B7-H3, LILRB2, VISTA, BTLA are also popular targets that many pharmaceutical company are working on.
l TIM-3 (T-cell immunoglobulin-3) is a T-cell surface suppressor molecule that causes T-cell exhaustion during cancer and chronic viral infections. According to Pharnexcloud database, there are 46 TIM-3 targeted drugs in development worldwide, including TIM-3/PD-1 targeted bispecific antibodies (such as Roche's RG-7769 and AstraZeneca's AZD-7789), among which Novartis' sabatolimab is the most advanced and is already in Phase III clinical. It is a TIM-3-targeted humanized IgG4k antibody developed by Novartis for the treatment of myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML), and was granted the orphan drug by EC for the treatment of MDS.
l TIGIT (T-cell immunoglobulin and ITIM structural domain), is an inhibitory receptor shared by T cells and natural killer cells (NK cells), which inhibits their killing effect on tumor cells. According to Pharnexcloud database, there are 59 TIGIT-targeted drugs in the world, and many of them are in Phase III clinical trials, such as BeiGene's ociperlimab, Roche's tiragolumab, Gilead/Arcus Bioscience's domvanalimab, MSD's MK-7684A (compound preparation of vibostolimab + pembrolizumab).
l 4-1BB, also known as CD137, is a T-cell co-stimulatory molecule and an important member of the tumor necrosis factor receptor (TNF) superfamily, mainly expressed on activated CD4+ and CD8+T cells, and natural killer cells (NK cells). At the moment, global pharmaceutical companies have developed more than 100 drugs around 4-1BB target, with their main types being monospecific antibody and bispecific antibody. Utomilumab, developed by Pfizer, is in relatively fast progressing and is in Phase III clinical trials. Utomilumab is a humanized IgG2 monoclonal antibody with a different mechanism of action from BMS' Urelumab, which can activate 4-1BB and block binding to 4-1BBL. Therefore, it has a relatively mild agonistic ability on immune cells and a high safety profile. Utomilumab is currently being explored in combination with PD-1 inhibitors, and the results of a Phase Ib clinical trial of the drug in combination with a K-drug, published in 2016, showed that: the overall ORR of the combination therapy for solid tumors was 26% (6/23).
B7-H3 (CD276) is a type I transmembrane protein, a member of the B7 immune co-stimulatory and co-inhibitory family. Increasing evidence suggests that B7-H3 plays a role in immune cells primarily as a co-inhibitor, facilitating the evasion of tumor cell from immune surveillance. 38 drugs have been developed worldwide based on B7-H3 target, with Y-mAbs Therapeutics' omburtamab being the most advanced, and BLA of which for the treatment of pediatric patients with neuroblastoma in central nervous system (CNS)/leptomeningeal metastasis has been submitted. The drug is a B7-H3-targeted monoclonal antibody that is radiolabeled prior to intracerebroventricular central nervous system (CNS) administration. Omburtamab was developed by researchers at Memorial Sloan-Kettering Cancer Center (MSK) in the US and licensed exclusively by MSK to Y-mAbs Therapeutics. SciClone Pharmaceuticals acquired exclusive co-development and commercialization rights for omburtamab and Danyelza (GD2-targeted monoclonal antibody) in the Greater China through a license agreement with Y-mAbs Therapeutics in December 2020.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


