LianBioMarch 31, 2022
Tag: LianBio , Mavacamten , TP-03
Phase 3 registrational trial of mavacamten in Chinese patients with symptomatic obstructive hypertrophic cardiomyopathy (oHCM) ongoing
Mavacamten granted breakthrough therapy designation in China for the treatment of patients with oHCM
Infigratinib approved under special named patient program for the treatment of cholangiocarcinoma (CCA) in the Bo'Ao pilot zone of Hainan Province in China
Three additional pipeline programs expected to enter into registrational Phase 3 clinical trials in China by year-end 2022
Cash balance of $403.2 million with runway through 2023
LianBio (Nasdaq: LIAN), a biotechnology company dedicated to bringing innovative medicines to patients in China and other major Asian markets, today reported financial results for the fourth quarter and year ended December 31, 2021.
“We are proud of our progress over the past year, highlighted by the achievement of key clinical and corporate milestones, including the initiation of the Phase 3 EXPLORER-CN trial of mavacamten in Chinese patients with symptomatic oHCM, and the approval of infigratinib for the treatment of cholangiocarcinoma patients in the Hainan Province in China,” said Yizhe Wang, Ph.D., Chief Executive Officer of LianBio. “LianBio continues to advance our pipeline of clinically validated therapeutic candidates and we plan to initiate pivotal studies of three additional programs, TP-03, NBTXR3 and infigratinib, in China by year-end. We remain focused on our cross-border development strategy and are committed to building our pipeline of novel medicines for patients in Greater China and other Asian markets.”
Recent Business Highlights and Clinical Development Updates
Phase 3 EXPLORER-CN trial of mavacamten in symptomatic oHCM patients initiated and PK trial dosing completed
• In November 2021, LianBio initiated and completed enrollment and dosing in a pharmacokinetic (PK) study of mavacamten in healthy Chinese volunteers.
• In January 2022, LianBio initiated the Phase 3 EXPLORER-CN clinical trial of mavacamten in Chinese patients with symptomatic oHCM.
• In February 2022, LianBio's partner Bristol Myers Squibb (BMS) announced positive topline results from the Phase 3 VALOR-HCM trial, evaluating mavacamten in patients with oHCM who are eligible for septal reduction therapy.
• In February 2022, LianBio announced that the Center for Drug Evaluation (CDE) of the National Medical Products Administration (NMPA) granted Breakthrough Therapy Designation in China for mavacamten for the treatment of patients with oHCM.
Infigratinib approved for the treatment of CCA in the Bo'ao pilot zone of Hainan Province
• In December 2021, infigratinib received approval from the Health Commission and Medical Products Administration of Hainan Province, under the special Named Patient Program (NPP), for the treatment of patients with previously treated, unresectable locally advanced or metastatic cholangiocarcinoma with a FGFR2 fusion or other rearrangement. The approval enables early commercial access to infigratinib in the Bo’ao Lecheng International Medical Tourism Pilot Zone based on the drug’s approval in other global jurisdictions.
Expanded leadership team with key executive hires and named two additions to board of directors
• In October 2021, LianBio named Michael Humphries, MBBS, as Chief Scientific Advisor to guide research and development (R&D) strategy, advance the pipeline, and lead assessment of new in-licensing opportunities.
• In October 2021, LianBio appointed Jesse Wu and Susan Silbermann to the Board of Directors.
Raised $334.5 million of gross proceeds from IPO
• In November 2021, LianBio raised gross proceeds of $334.5 million and aggregate net proceeds of $304.8 million in connection with its initial public offering and subsequent exercise of the underwriters’ option to purchase additional ADSs.
Mavacamten
BMS-partnered cardiac myosin inhibitor in development for the treatment of hypertrophic cardiomyopathy and certain forms of heart failure
• LianBio’s partner BMS has announced a Prescription Drug User Fee Act (PDUFA) target action date of April 28, 2022 for its New Drug Application to the U.S. Food and Drug Administration (FDA) for mavacamten for the treatment of patients with oHCM.
TP-03
Tarsus Pharmaceuticals-partnered GABA-Cl channel blocker in development for the treatment of Demodex blepharitis (DB) and meibomian gland disease
• LianBio expects to initiate a Phase 3 trial of TP-03 in Chinese patients with DB in the second half of 2022 to support regulatory approval in China.
• LianBio’s partner Tarsus has announced that it expects to report topline data in April 2022 from the ongoing Phase 3 Saturn-2 trial of TP-03 in DB patients.
NBTXR3
Nanobiotix-partnered radioenhancer in development for multiple solid tumor indications
• LianBio expects to begin dosing Chinese patients in Nanobiotix’s ongoing global pivotal Phase 3 trial of NBTXR3 for the treatment of locally advanced head and neck squamous cell carcinoma in elderly patients ineligible for cisplatin in the second half of 2022.
QED-partnered FGFR1-3 inhibitor in development for the treatment of individuals with FGFR-driven cancers
• LianBio expects to begin dosing Chinese patients in QED’s ongoing global pivotal Phase 3 PROOF-301 trial of infigratinib in first-line CCA patients with FGFR2 gene fusions/translocations in the second half of 2022.
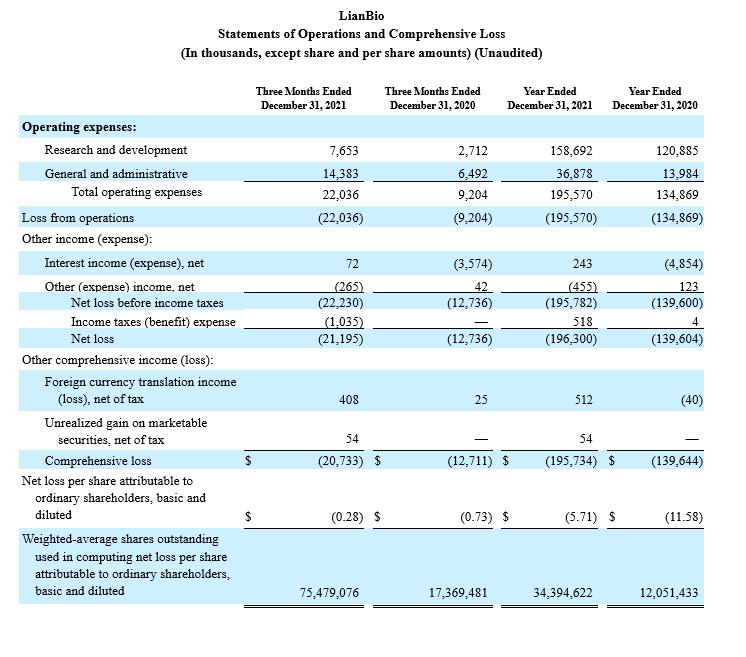
R&D Expenses: Research and development expenses were $7.7 million for the fourth quarter of 2021 compared to $2.7 million for the fourth quarter of 2020, and $158.7 million for the year ended December 31, 2021 compared to $120.9 million for the year ended December 31, 2020. The increase was primarily attributable to increased milestone payments and development activities to support clinical trials and personnel-related expenses (including share-based compensation expense) as a result
of increased employee headcount, development activities to support our clinical trials and professional
fees.
G&A Expenses: General and administrative expenses were $14.4 million for the fourth quarter of 2021 compared to $6.5 million for the fourth quarter of 2020, and $36.9 million for the year ended December 31, 2021 compared to $14.0 million for the year ended December 31, 2020. The increase was primarily attributable to increases in payroll and personnel-related expenses (including share-based compensation expense) for increased employee headcount and increases in legal service costs, consulting costs and accounting services.
Net Loss: Net loss was $21.2 million for the fourth quarter of 2021 compared to net loss of $12.7 million for the fourth quarter of 2020, and $196.3 million for the year ended December 31, 2021 compared to $139.6 million for the year ended December 31, 2020.
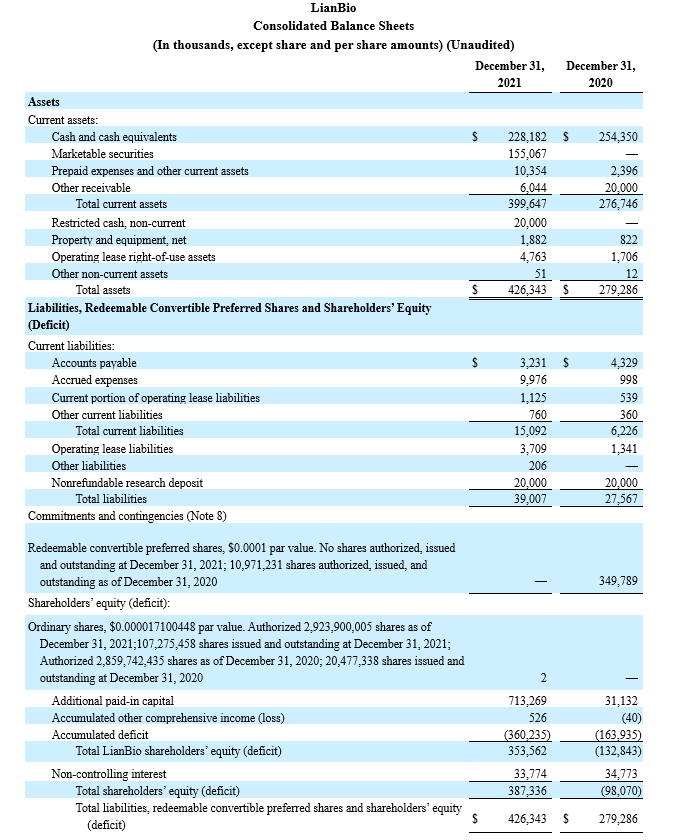
Cash balance: Cash, cash equivalents, marketable securities, and restricted cash at December 31, 2021 totaled $403.2 million, reflecting a net increase of $148.9 million from December 31, 2020. LianBio projects its cash position is sufficient to fund current operations through 2023.
LianBio is a cross-border biotechnology company on a mission to bring transformative medicines to historically underserved patients in China and other Asian markets. Through partnerships with highly innovative biopharmaceutical companies around the world, LianBio is advancing a diversified portfolio of clinically validated product candidates with the potential to drive new standards of care across cardiovascular, oncology, ophthalmology, inflammatory disease and respiratory indications. LianBio is establishing an international infrastructure to position the company as a partner of choice with a platform to provide access to China and other Asian markets.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


