David Orchard-WebbMarch 16, 2022
Tag: Oncology , Vaccines , HIV
The last ten biopharma companies that have IPO’d on the STAR Market (Shanghai Stock Exchange Science and Technology Innovation Board) are focused on oncology, synthetic biology, genomics, HIV & pain management, vaccines, antibodies, and other biologics and small molecules. Figure 1 shows the global pharmaceutical market share of these categories as of 2020. Biologics and small molecules combined represent the majority of the biopharmaceutical market.
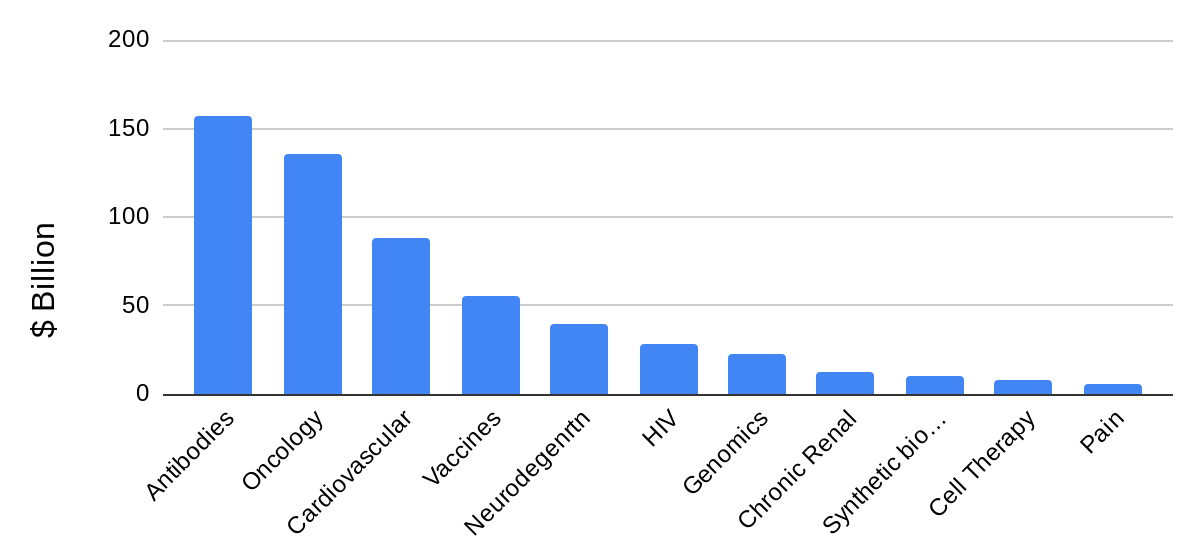
Figure 1: 2020 global market share vs. category.
Four of the last ten companies to IPO are large generalist biopharmaceutical companies that cover the full range of therapeutics and disease indications. One of the companies is focused on the major antibody market. The remaining companies were diverse focussing on different aspects of the aforementioned categories.
Sinopep is a market-oriented, innovation-driven biopharmaceutical company. The R&D, manufacturing and sales is of both peptide and small molecule pharmaceuticals. Over more than 11 years, the company has successfully developed five technology platforms; 1) large scale solid-solution phase hybrid production technology, 2) chiral-drug design and selection technology, 3) continuous-flow microchannel green-chemistry technology, 4) drug delivery technology, 5) new peptide drug design technology.
Sinopep has an extensive IP portfolio with 53 patents for various inventions, which are widely used in the research, development and production for the company's products. The company produces a wide range of products for indications such as diabetes, cardiovascular, oncology, antiviral, and assisted fertility.
Sinopep’s key products are liraglutide, semaglutide, thymalfasin, eptifibatide, bivalirudin, octreotide acetate, lanreotide acetate, cetrorelix, fulvestrant, oseltamivir.
Headquartered in Beijing, Youcare Pharmaceutical Group was founded in 2001. Youcare has established a pharmaceutical research institute in Beijing, and different manufacturing bases in Beijing, Guangzhou, Anhui, and Henan.
The company follows clinical needs as guidance. Youcare has consecutively established the National and Local Joint Engineering Laboratory for Cephalosporin Crystal Form Research, Beijing Engineering Research Center for Cardio-cerebral Vascular Medicine and the Technological Development Platform for Micro-pellet Sustained-Release and Controlled-Release Preparations, and established a Youcare Group Academic Expert Workstation, and Postdoctoral Research Station.
The company currently has over 200 drug products, covering multiple therapeutic fields including cardio-cerebral vascular drugs, gastrointestinal drugs,anti infective agents, endocrine drugs and antineoplastic drugs.
Kexing Biopharmaceutical Co., Ltd., engages in the research and development, production, and sale of recombinant protein therapeutics and microbiota agents. The company was founded in 1997 and is based in Jinan, China. Its pharmaceutical research focuses on the therapeutic fields of antiviral, hematology, oncology, and immunology, as well as degenerative diseases. The company’s products include recombinant human interferon a1b, recombinant human erythropoietin, and recombinant human granulocyte colony-stimulating factor injections, as well as combined clostridium butyricum and bifidobacterium capsules.
Founded in 2009 , Easton Biopharmaceuticals is a high-tech enterprise integrating R&D, production and sales of chemical raw materials, high-end chemical medicines and biopharmaceuticals with approximately 1,000 employees.
Taking on the “Healthy China Initiative”, the company is devoted to drug research and development, focused on major diseases such as anesthesiology & pain management, cardiovascular, oncology, children's medicines, and diabetes. By the end of April 2021, it had industrialized 26 high-end chemical drugs and 17 chemical material medicines. Easton Biopharmaceuticals had the first four generic drugs for the Chinese market, fourteen generic drugs have passed Quality Consistency Evaluation for Generic Drugs (QCE), and five chemical material medicines have been certified in mainstream international markets such as the European Union, Japan, and the United States.
Easton Biopharmaceuticals continues to maintain high R&D investment, which accounted for more than 16% of expenses from 2017 to 2020, much higher than industry average in China. Easton Biopharmaceuticals has a strategy based on high-end generic drugs, focusing on small-molecule innovative drugs, and seeking the development of biological drugs. The company has 10 innovative drugs under development, of which 2 have entered clinical trials in phase I and II respectively, and many biological drugs under preclinical study.
Founded in 2001, Wuhan Keqian Biological Co., Ltd. is focused on the development, production, sales, veterinary technical services, animal vaccines, diagnostic reagents, biological therapeutic agents, antibodies and feed additives.
The company is a high-tech enterprise established by scientific researchers from the Animal Infectious Diseases Laboratory of Huazhong Agricultural University. Keqian Bio has a research and development technical team led by academics and a high-quality management and operation team.
With the strategic goal of "building national industrial brand and leading industrial development", the company adheres to the values of "creating value through science and technology and repaying society with science and technology". Staff stick to the "innovation, creation, entrepreneurship" spirit, and the pursuit of "loyalty, enterprising, rigorous, pragmatic" attitudes.
Founded in 2004, Shanghai Allist Pharmaceuticals Co., Ltd. is a biotech with integrated capabilities in the R&D, manufacturing and commercialization of small-molecule medicine. Driven by innovation of R&D, Allist strives to develop the first-in-class and best-in-class drugs. The company has built a strong in-house drug R&D platform and successfully developed two new drugs in the past 16 years.
Allist has built a strong product pipeline focusing on the Non Small Cell Lung Cancer (NSCLC). Furmonertinib, its core product, is a 3G EGFR-TKI with strong performance in safety and efficacy both for primary lung cancer and brain metastasis. With the GMP compliant manufacturing facilities, Furmonertinib, has been commercialized in Mar 2021 for the indication of second-line treatment of EGFR+ NSCLC, and more indications of Furmonertinib are under development.
The ongoing phase Ib clinical trial of Furmonertinib has shown great potential for the treatment of NSCLC patients with EGFR Ex20ins mutation. The KRAS-G12C inhibitor and RET inhibitor under development are expected to enter clinical trials in 2022. Other pre-clinical products under development include KRAS G12D inhibitor, 4th-generation EGFR-TKI and SOS1 inhibitor.
The management team of Allist boasts a wealth of experience on EGFR-TKIs and PD-1/PD-L1 immune-checkpoint inhibitors. For the commercialization of Furmonertinib, Allist also built a professional medical, marketing and sales function team whose members have an industry-enviable and highly focused oncology expertise, especially in the NSCLC area in China.
Want to get more information on pharma, like where to find trustable hospital medical supply companies? Pharmasources could help you!
Founded in 2009, based in Tianjin, CanSino Biologics Inc. is an innovative biopharmaceutical company dedicated to exploring best solutions to the prevention of diseases through cutting-edge research & development, advanced manufacturing and commercialization of innovative vaccine products for human use worldwide.
CanSinoBIO has experienced tremendous growth with 17 vaccines preventing 12 diseases, including approved vaccines for Ebola virus disease (Ad5-EBOV), COVID-19 (Ad5-nCoV, trade name: Convidecia), and meningitis (MCV2, trade name: Menphecia). CanSinoBIO is focusing on continually expanding manufacturing capacity for its current vaccine candidates and further enhancing the competitiveness and the scope of its portfolio by promoting the R&D of new vaccine candidates.
The company leverages the broad experience of their accomplished team of scientists and business leaders who had previously held technical and senior management positions at many of the leading pharmaceutical companies in the world, including Sanofi Pasteur, AstraZeneca, Wyeth (now Pfizer) and CNBG (China). The company has developed five key platform technologies, including viral vector-based technology, synthetic vaccine technology, protein structure design and recombinant technology, mRNA technology as well as formulation and delivery technology.
The Recombinant Novel Coronavirus (SARS-CoV-2) vaccine using an Adenovirus type 5 vector backbone, called Convidecia was approved by NMPA of China, making it the first of its kind authorized in China. Ad5-EBOV, the globally innovative Ebola virus disease vaccine, had received NDA (New Drug Application) approval in China in October 2017. The Group A and Group C Meningococcal Conjugate Vaccine (CRM197) and the Group ACYW135 Meningococcal Conjugate Vaccine (CRM197) were approved in 2021.
At present, CanSinoBIO has six vaccine candidates in the clinical trial stage or new drug application stage. There are also seven preclinical vaccine candidates under development, including one combination vaccine candidate.
Founded in 2013, Frontier Biotechnologies Inc. is a commercial-stage biopharmaceutical company headquartered in China. The company is committed to discovery, development, manufacturing, and commercialization of innovative medicines that improve patient health. Frontier’s medicines typically address the unmet medical needs of patients in the areas of anti-HIV treatment and pain management.
Frontier Biotech's first commercial product was approved by the National Medical Product Administration (NMPA), which is the counterpart of the Food and Drug Administration (FDA) in China, in May 2018 through a priority review process and gained accelerated approval. Two additional drug candidates are currently undergoing multiple clinical trials in Phase I and Phase II in both China and the United States.
Frontier Biotech was co-founded by three nationally distinguished experts, Dr. Dong Xie, Dr. Changjin Wang, and Dr. Rongjian Lu, making Frontier one of the leading biotechnology companies in China in the field of innovative drug development for HIV and AIDS treatments.
Frontier Biotech has been the recipient of numerous awards and accolades over the years, named as the leading enterprise in the field of Anti-HIV Drug Development Special Project in the 13th "Five-Year Plan" New Drug Research and Development National Major Scientific and Technological Special Projects administered by State Ministry of Science and Technology. Frontier Biotech is also named as one of the "Cultivation of Unicorn Enterprises" in the city of Nanjing, China.
Founded in March 2011, headquartered in Beijing, Novogene is specialized in the application of advanced molecular biotechnology high-performance computing in the life science and human health fields. It strives to become a global leading provider of genetic science and technology product and services. The company has established high-flux large-scale gene sequencing and high-performance computing platforms, which effectively support the demands of life science research and medical health for big data analysis and storage.
As a leader in the gene sequencing field of China, Novogene's business covers the fundamental scientific research service of life science, medical research and technology service, storage and sequencing platform services, providing gene sequencing, mass spectrometry and bioinformatics technology support for global research universities, scientific research institutions, hospitals, pharmaceutical R&D enterprises and agricultural enterprises.
Novogene has established extensive cooperation with many academic institutions all over the world, and completed several genomics research projects at an internationally advanced level. By October 2020, Novogene published more than 600 science articles in cooperation with project partners, accumulating an impact factor of over 4270. Currently, it has obtained 208 software copyrights and 36 patents. It has partners all over the world, including over 2,600 scientific research institutions and universities, over 590 hospitals, over 1,200 pharmaceutical and agricultural enterprises.
Established in 2005, Anhui Huaheng Biotechnology Co., Ltd., has synthetic biotechnology as its core enterprise, mainly engaged in the R&D, production and sales of amino acids and their derivatives. Anhui Huaheng has successfully undertaken key national science and technology projects and high-tech industrialization projects such as the "synthetic biology" key project of the Ministry of science and technology, the biological industry demonstration project of the national development and Reform Commission, and won the single champion product of the Ministry of industry and information technology, the patent advantage enterprise of the State Intellectual Property Office, and the China Patent Excellence Award.
The main products of Anhui Huaheng include alanine series products (L-alanine, DL alanine β- Alanine, L-valine, d-pantothenate calcium, d-pantothenol and L-valine α- Arbutin. The products are mainly used in medicine and health products, food and feed additives and other fields.
Anhui Huaheng adheres to the concept of green environmental protection, thrives on technological breakthroughs and generates cost advantages, and has long served many of the world's top 500 and industry leading enterprises, including BASF.
The last ten biopharmaceutical IPOs on the Shanghai STAR market have been diverse, with the majority in the broad biopharmaceutical category. Big markets such as antibodies, vaccines, HIV and genomics were also addressed. Contrasting this there have been some very focused companies in oncology such as Shanghai Allist, exclusively targeting NSCLC. Anhui Huaheng Biotech Co Ltd is also very specialized in the synthetic biology of amino acids. This mix of generalists and specialists has made the last ten IPOs on the STAR market very competitive.
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


