PharmaSources/RecollectFebruary 22, 2022
Tag: COVID-19 Vaccine , Therapeutic Drugs , FDA , NMPA
Since the outbreak of COVID-19 in 2019, many kinds of COVID-19 vaccines, COVID-19 preventive drugs and COVID-19 therapeutic drugs have been approved worldwide. The approved COVID-19 vaccines include inactivated vaccine, adenovirus vaccine, recombinant protein vaccine, mRNA vaccine and DNA vaccine, and the therapeutic drugs include chemical drugs, antibodies and Chinese patent medicines. Next, I will summarize the approved COVID-19 vaccines and therapeutic drugs in the U.S. and China.
Up to now, FDA has approved at least 3 types of COVID-19 vaccines (2 mRNA vaccines and 1 adenovirus vaccine), 1 pre-exposure preventive drug and 9 COVID-19 therapeutic drugs. Jointly developed by AstraZeneca and Vanderbilt University Medical Center, Evusheld is a long-acting neutralizing antibody combination therapy, which consists of two neutralizing antibodies tixagevimab and cilgavimab that bind different epitopes of SARS-CoV-2 spike protein. In December 2021, it was granted the emergency use authorization (EUA) by FDA for pre-exposure prophylaxis (PrEP) of SARS-CoV-2 infection (COVID-19) in specific adults and adolescents (aged ≥ 12 years old and weighted ≥ 40kg). It is currently the only authorized pre-exposure prophylaxis therapy for COVID-19 in the U.S.
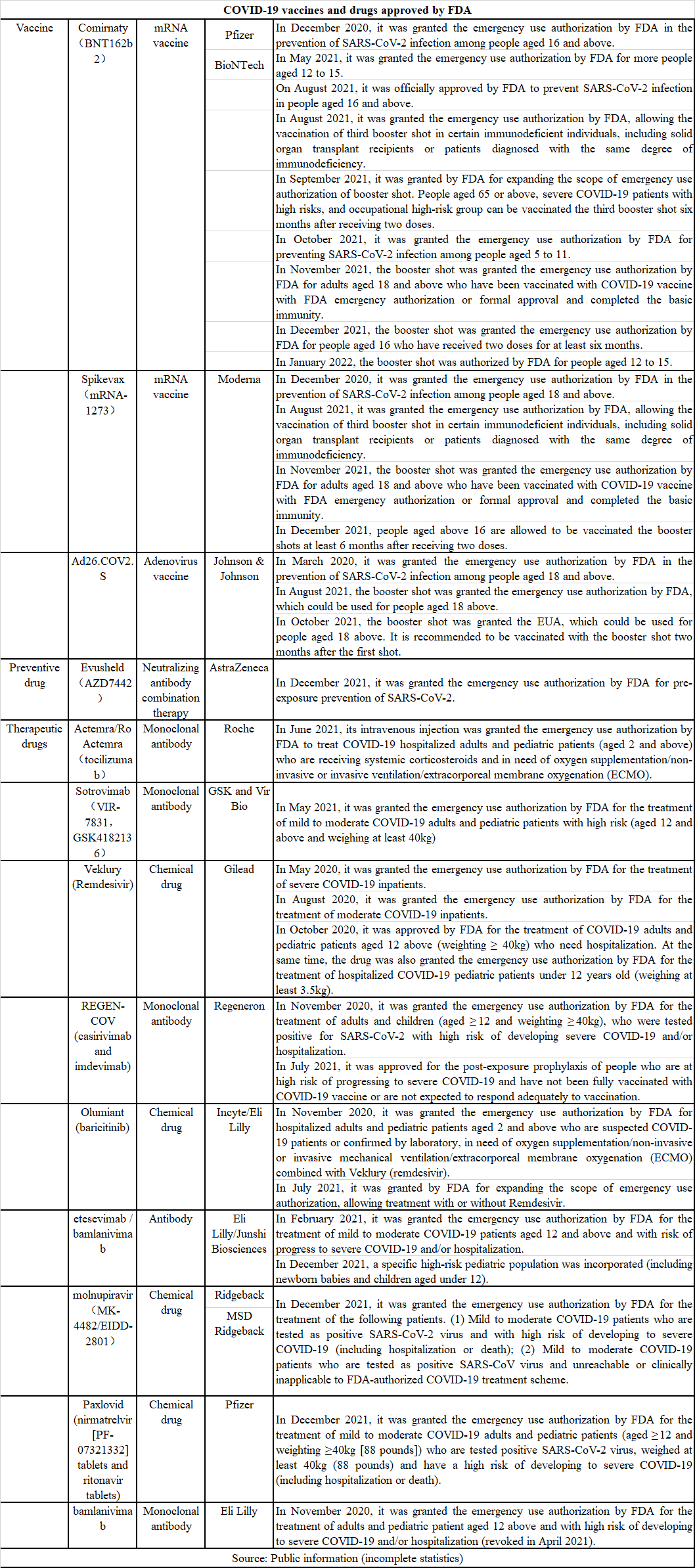
Actemra/RoActemra is the first humanized monoclonal antibody drug against IL-6 receptor (IL-6R) in the world, which can play an anti-inflammatory role by blocking IL-6 signal transduction by targeted binding with IL-6R. It has been approved for the treatment of rheumatoid arthritis (RA), polyarticular juvenile idiopathic arthritis (pJIA), systemic juvenile idiopathic arthritis (sJIA), giant cell arteritis (GCA), cytokine release syndrome (CRS), Castleman's disease, Takayasu's arteritis and systemic sclerosis-related interstitial lung disease. In June 2021, Actemra/RoActemra intravenous injection was granted the emergency use authorization by FDA to treat COVID-19 hospitalized adults and pediatric patients (aged 2 and above) who are receiving systemic corticosteroids and in need of oxygen supplementation/non-invasive or invasive ventilation/extracorporeal membrane oxygenation (ECMO).
Sotrovimab is a neutralizing antibody jointly developed by GlaxoSmithKline (GSK) and Vir Biotechnology, which has dual functions and can block viruses from entering healthy cells and clear infected cells at the same time. Sotrovimab can bind to an epitope shared by SARS-CoV-2 and SARS-CoV-1 (the virus causing SARS), which is highly conserved and may make it more difficult to produce drug resistance. In addition, with the use of Xencor's Xtend technology, sotrovimab is also designed to achieve high concentration in the lungs to ensure optimal penetration of airway tissue affected by SARS-CoV-2 and a prolonged half-life. In May 2021, it was granted the emergency use authorization by FDA for the treatment of mild to moderate COVID-19 adults and pediatric patients with high risk (aged 12 and above and weighing at least 40kg), which includes: patients with positive results of direct SARS-CoV-2 virus test and at high risk of developing into severe COVID-19 (including hospitalization and death).
REGEN-COV is a kind of cocktail therapy, which was firstly invented by Regeneron, and jointly developed by Regeneron and Roche. It is composed of casirivimab and imdevimab monoclonal antibodies, which target two independent and non-overlapping sites in the receptor binding region of spike (S protein) in SARS-CoV-2, and have synergistic effect. It can reduce the risk of virus mutation escaping and protect people from invasion of virus variants with mutation of S protein. Preclinical research data show that the neutralizing activity of casirivimab and imdevimab is kept, which is used to fight against key emerging variants. In November 2020, REGEN-COV was granted the emergency use authorization by FDA for the treatment of mild to moderate COVID-19 children (age ≥ 12 and weighting ≥ 40kg) and adults with positive SARS-CoV-2 virus test results and high risk of developing to severe COVID-19 patients and/or hospitalization. In July 2021, the FDA updated the EUA of REGEN-COV to allow post-exposure prophylaxis for people with high risk of developing to severe COVID-19. It means that REGEN-COV is the first COVID-19 neutralizing antibody licensed in the U.S. for both the treatment of COVID-19 patients and post-exposure prophylaxis.
The active pharmaceutical ingredient of Olumiant is baricitinib, which is a selective and reversible JAK1 and JAK2 inhibitor taken orally once a day. It is developed for the treatment of various inflammatory and autoimmune diseases. In November 2020, it was granted the emergency use authorization by FDA for hospitalized adults and pediatric patients aged 2 who are suspected COVID-19 patients or confirmed by laboratory, in need of oxygen supplementation/non-invasive or invasive mechanical ventilation/extracorporeal membrane oxygenation (ECMO) combined with Veklury (remdesivir). In July 2021, it was granted by FDA for expanding the scope of emergency use authorization, allowing treatment with or without Remdesivir.
Paxlovid and Molnupiravir are both oral COVID-19 drugs, among which the former was granted the emergency use authorization by FDA on December 22, 2021. Paxlovid is used to treat adults and children (aged ≥12 and weighting ≥40kg or about 88 pounds) with mild to moderate COVID-19, as well as people with high risk of severe illness. It has become the first oral SARS-CoV-2 therapeutic drug authorized by FDA. Molnupiravir was granted the emergency use authorization (EUA) by FDA on December 23, 2021 for the treatment of adults with mild to moderate SARS-CoV-2 virus direct test results and potential severe risk factors (including hospitalization or death), and patients who are not available or clinically not apply other COVID-19 alternative treatment schemes approved by FDA.
Bamlanivimab is a potent and neutralizing IgG1 monoclonal antibody against SARS-CoV-2 spike protein. The R&D of this drug is aimed to prevent the virus from attaching and entering human cells, thereby neutralizing the virus and potentially preventing and treating COVID-19 patients. In November 2020, Bamlanivimab was granted the emergency use authorization by FDA, and it could be used as a single drug to treat mild and moderate COVID-19 patients aged 12 and above with high risk of progression to severe or hospitalization. However, in April 2021, FDA revoked the EUA, because it was found that drug resistance would occur when a single drug was used for SARS-CoV-2 variant virus. FDA believed that if the drug was used in the future, the potential risks would outweigh the benefits. Etesevimab is a recombinant humanized monoclonal neutralizing antibody, which specifically binds to the binding domain of spike protein receptor on SARS-CoV-2 surface with high affinity, and can effectively block the binding of virus to host cell surface receptor ACE2. In February, 2021, the combination therapy of Etesevimab and Bamlanivimab was granted the emergency use authorization by FDA, which is used to treat mild and moderate COVID-19 patients aged 12 and above with high risk of progression to severe disease or hospitalization. In December 2021, the emergency use authorization (EUA) was expanded by FDA again, and the applicable population was extended to specific high-risk pediatric population (including newborn babies and children aged under 12). It is worth mentioning that Etesevimab/Bamlanivimab therapy is the first and only neutralizing antibody therapy covering children aged under 12 in the world.
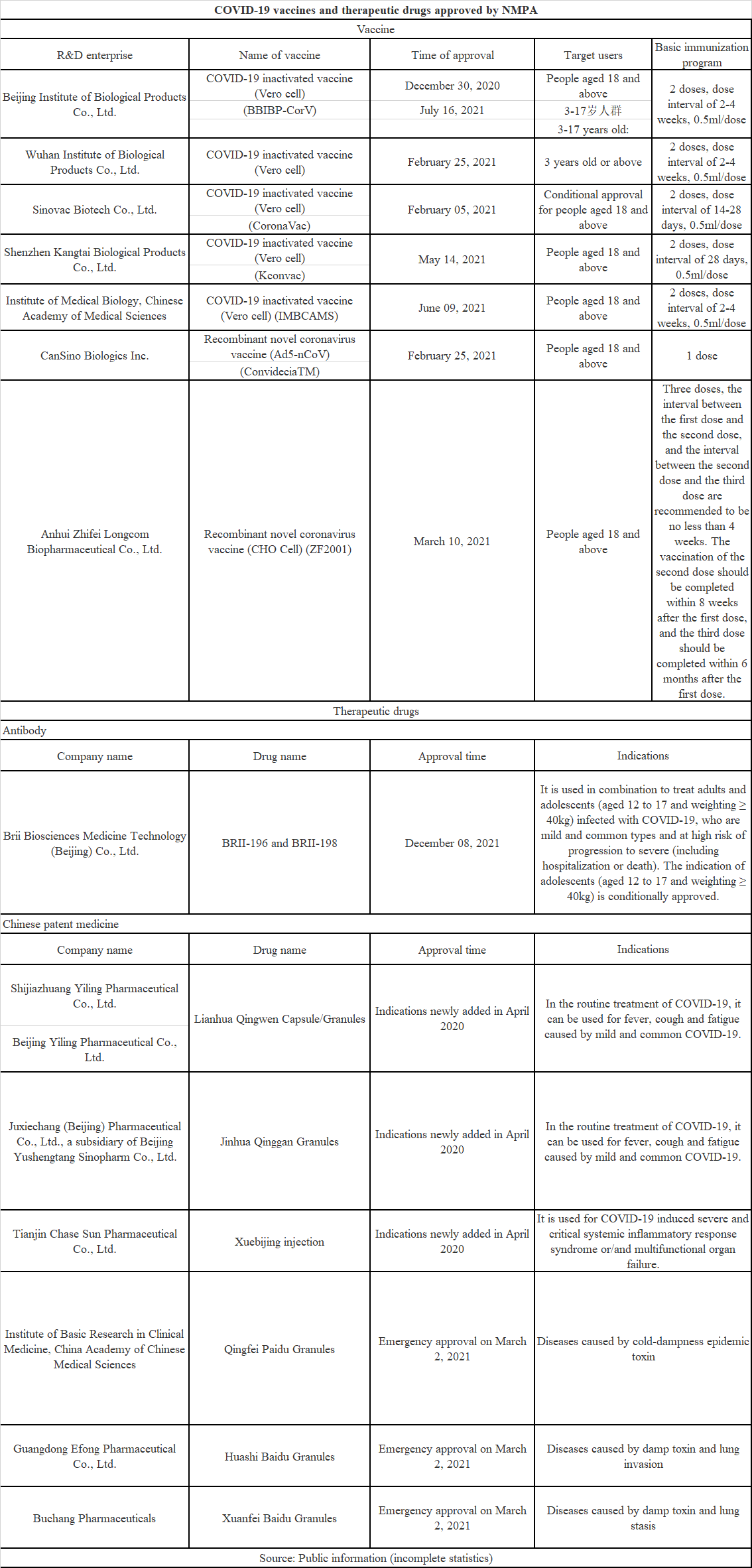
Up to now, according to incomplete statistics, Chinese NMPA has approved at least 7 vaccines, 6 traditional Chinese medicines and 1 neutralizing antibody, as shown in the following table. The approved COVID-19 vaccines include inactivated vaccine, adenovirus vaccine and recombinant protein vaccine. BRII-196/BRII-198 of Brii Biosciences is the first COVID-19 specific drug approved by China, and the emergency use authorization for this therapy has been submitted to FDA in October 2021.
In addition, it is worth mentioning that in addition to the approved COVID-19 vaccine mentioned above, India has also approved a DNA COVID-19 vaccine - ZyCoV-D. It was developed by an Indian pharmaceutical company, Zydus Cadila. The vaccine applies circular DNA strands to stimulate the immune system against SARS-CoV-2. Different from other COVID-19 vaccines, ZyCoV-D is administered by a needle-less device instead of syringe needle, which can generate tiny high-pressure fluid and directly penetrate the skin surface, thus avoiding the pain caused by acupuncture.
Although a variety of COVID-19 vaccines and therapeutic/preventive drugs have been approved in the world, with the mutation of SARS-CoV-2, it is still a tough task faced by human beings. It is expected that more effective vaccines and drugs against COVID-19 will be developed in the future to end the pandemic.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


