PharmaSources/YiJanuary 13, 2022
Tag: Aurora Kinase Inhibitors , Aurora A , Aurora B
Aurora kinase is the serine/threonine proteases engaged in cell mitosis, primarily serving at the centriole and spindle to support the regular cell division. In 1995, Professor Glover and his team at the Centre for Anatomy and Human Identification at Dundee University in Scotland revealed that the enzyme is involved in the process of cell division in Drosophila cells. However, in cases where this enzyme is mutated, the centrioles will not separate, resulting in what is known as a "unipolar spindle" morphology, which interferes with cell division and the eventual formation of polyploids.
There are three isoforms of human Aurora kinase, A, B and C. Aurora A and Aurora B are expressed in many cells of human body whereas Aurora C is found mainly in testicular tissues. Typically, Aurora A exerts its action during the G2/M phase of mitosis, influencing such processes as centriole maturation and spindle formation. However, in tumor cells, Aurora A is expressed during the whole cycle of cell division, which affects the function of other proteins in the cytoplasm, e.g. inhibiting the activity of oncoproteins like p53, BRCA1, Chfr and resulting in tumor cell production.
Multiple Aurora kinase inhibitors are currently in development worldwide, which are detailed in the table below, mainly Aurora A inhibitors. And if you want to know more about medical products supply, Pharmasource would be your best choice.
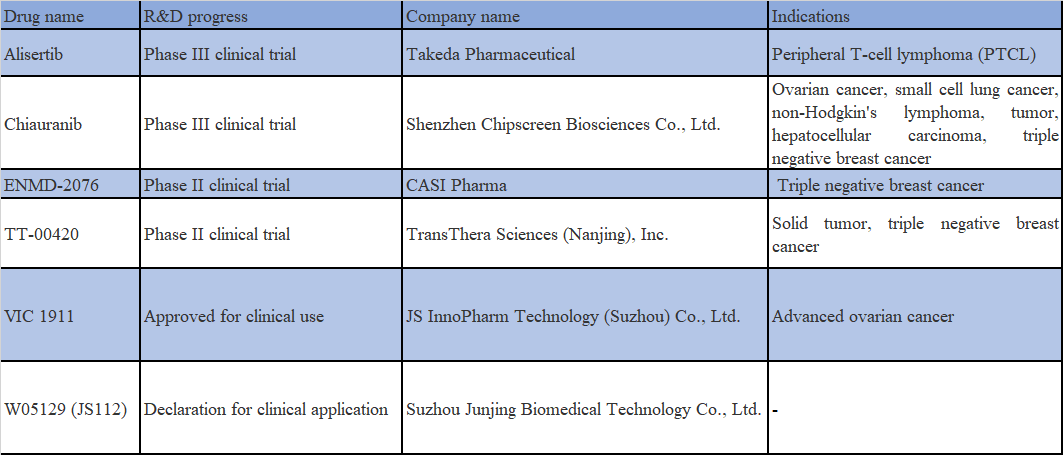
Alisertib is a selective Aurora kinase inhibitor with an ORR of 21% for single-agent Alisertib in a phase-1/2 basket trial comprising 48 cases of SCLC. However, the results of another phase-2 study examining the efficacy and safety of paclitaxel ± Alisertib in the second-line treatment of SCLC demonstrated that the combination treatment group showed numerical improvement in both PFS and OS, although the difference was not statistically significant. Besides, the incidence of adverse reactions and discontinuation of the drug is at a higher rate in the combination treatment group.
Chiauranib is a new chemical structure with global patent protection designed and developed by Chipscreen, which is a multi-target protein kinase inhibitor. Through the selective inhibition of multiple kinase targets, including Aurora B, CSF1R and VEGFR/PDGFR/c-Kit, it inhibits tumor cell proliferation, fosters anti-tumor immunity and inhibits tumor angiogenesis to maximize the anti-tumor efficacy of multiple pathways. The basis for the efficacy of Chiauranib as a single agent in the treatment of small cell lung cancer (SCLC) is demonstrated through the inhibition of molecular mechanisms associated with the potentially aberrantly active Aurora B pathway in small cell lung cancer. Last December, this drug was included by the CDE as a breakthrough therapy for the treatment of SCLC with disease progression or relapse after a 2nd-line systemic chemotherapy regimen.
ENMD-2076 promotes high expression of chemokine genes CXCL10 and CXCL11 in tumor cells by targeting Aurora A and inhibiting its activity, which in turn leads to a decrease in STAT3 phosphorylation levels. It was found that the combination of ENMD-2076 with PD-1 antibody was more effective in inhibiting the growth of TNBC in animal models and boosting the response rate of tumor immunotherapy.
TT-00420 is a selective focused kinase inhibitor developed by TransThera, which shows dual mechanisms to inhibit tumor proliferation by inhibiting Aurora A and B related to cell cycle and tyrosine kinase, a receptor involved in angiogenesis. Abundant preclinical studies have indicated that TT-00420 has excellent inhibitory effect on malignant tumors, specifically on triple negative breast cancer transplantation tumor model. It is currently in a phase-I clinical research for the treatment of triple negative breast cancer (TNBC).
VIC-1911 is an Aurora A kinase inhibitor developed by Taiho in Japan. In June 2020, JS InnoPharm was granted an exclusive worldwide license to develop and commercialize the drug in the oncology field. Data from a phase-1a clinical trial completed in the United States indicated that the combinations of VIC-1911 and EGFR inhibitors, such as Osimertinib, are expected to deliver improved first-line therapeutic effect in EGFR-mutated NSCLC.
WJ05129 is an oral small molecule Aurora A inhibitor developed by Wigen Biomedicine. In 2020, Wigen Biomedicine entered into a technology transfer and collaboration agreement with Junshi Biosciences, transferring a 50% interest in four drug projects, including WJ05129, to Junshi Biosciences, and the two invested jointly to establish Junjing Biosciences.
However, the R&D of Aurora kinase inhibitors is not smooth. Over the past decade, more than 20 Aurora A kinase inhibitors have been ceased to be clinically available owing to toxic side-effects, like QT interval prolongation, bone marrow suppression, drowsiness and limited patient benefit. However, a rising number of researches in recent years have shown that Aurora A inhibitors have the potential to address many unmet clinical needs. For example, the Aurora A inhibitor LY3295668 could target at breast cancer patients with RB1 deletion mutations to exert a "synthetic lethal" effect and eliminate the problem of CDK4/6 inhibitor resistance. The activation of Aurora A is associated with acquired resistance to EGFR TKI therapy, which, if relieved, could also restore the sensitivity of resistant tumor cells to EGFR TKI.
In recent years, the R&D of Aurora kinase inhibitors continues. Along with independent R&D, several companies worldwide have also introduced Aurora kinase inhibitors from outside. For instance, Eli Lilly acquired Aurora A kinase inhibitor AK01 (LY3295668) through the acquisition of AurKa Pharma, and JS InnoPharm introduced Aurora A kinase inhibitor TAS-119 from Taiho, a Japanese antitumor drug company.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


