PharmaSources/YiDecember 02, 2021
Tag: AMX0035 , ALS treatments , NDA
On November 2, Amylyx Pharmaceuticals announced that it had submitted a new drug application of AMX0035 for the treatment of amyotrophic lateral sclerosis (ALS) to FDA.
Amyotrophic lateral sclerosis, commonly known as ALS, is a chronic progressive disease, which often involves upper motor neurons (brain, brainstem, spinal cord), lower motor neurons (cranial nucleus, spinal cord anterior horn cells) as well as the muscles of trunks, limbs and heads and faces controlled by lower motor neuron. Clinically, ALS is often manifested as mixed paralysis with damage of upper and lower motor neurons. Generally, the health of patients with ALS degenerates quickly, and they usually die of respiratory muscle paralysis within 3 to 5 years.
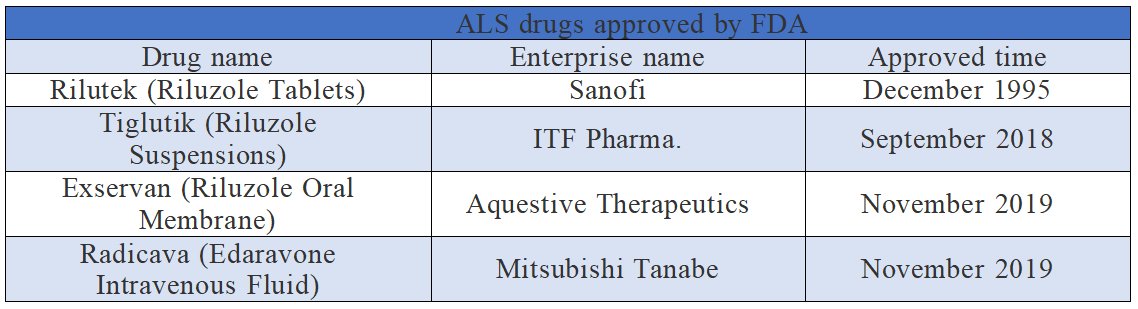
It is estimated that the incidence of ALS is about two per 100,000 people worldwide. About 5%-10% of ALS cases are related to heredity, but the cause of ALS is still unknown, which may be related to genetic and environmental factors. According to incomplete statistics, FDA has approved 4 drugs to treat ALS (see the table below for details). However these drugs can only slow down the development of ALS, instead of curing the disease.
Among them, it is thought the riluzole can reduce the damage to motor neurons by reducing the level of glutamic acid, which transmits information between nerve cells and motor neurons. Clinical trials have shown that riluzole can prolong the life of ALS patients by several months. Edaravone, the active drug component of Radicava, is a free-radical scavenger, which can inhibit lipid peroxidation, thus inhibiting the oxidative damage of brain cells, vascular endothelial cells and nerve cells. Its antioxidant effect can provide neuroprotective support for the nervous system, potentially delaying the development of diseases or limiting additional injuries.
AMX0035 is a combination of sodium phenylbutyrate (PB) and deoxycholic acid (TUDCA). In the experimental model, PB and TUDCA can alleviate endogenous endoplasmic reticulum stress and mitochondrial dysfunction, thus reducing neuronal death. In the preclinical research, Amylyx has proved that the combination of PB and TUDCA for the treatment of ALS had synergistic effect. It means that the therapeutic effect of combination of PB and TUDCA is better than using only one of them. In September 2017, FDA granted the it the qualification of orphan drug for the treatment of ALS.
The submission of AMX0035 NDA is based on the data of clinical research of CENTAUR. This research is a multi-center, randomized, double-blind clinical trial, including a 24-week placebo-controlled phase and an open-label follow-up phase. The main endpoint is to figure out the scale of decrease of ALSFRS-R (Amyotrophic Lateral Sclerosis Functional Rating Scale) compared with baseline at 24th week. A total of 137 ALS patients were enrolled in the research, and the patients were randomly assigned to AMX0035 group or placebo group according to the ratio of 2:1.
The results show that: at the 24th week, the average ALSFRS-S score of AMX035 group was 29.06, while that of placebo group was 26.73, with a difference of 2.33 points. After 24-week follow-up, the score of AMX0035 group was 2.92 points higher than the baseline. In addition, the results of three-year follow-up showed that compared with placebo, AMX0035 prolonged the overall median survival time of ALS patients (25 months vs 18.5 months) and reduced the risk of death by 44%.
In recent years, as many companies and medical supplies manufacturers have attached great importance to the orphan drug market, there are still many drugs for the treatment of ALS under research, such as Ultomiris (ravulizumab) of Alexion, arimoclomol of Orphazyme Company, masitinib of AB Science, MN-166 (ibudilast) of MediciNova and Cerebral Dopamine Neurotrophic Factor (CDNF) of Herantis.
Ultomiris is the first approved long-acting C5 complement inhibitor, which acts by inhibiting C5 protein at the end of complement cascade reaction. Complement cascade reaction is a part of immune system, and its uncontrolled activation plays an important role in severe rare diseases and super rare diseases. In January 2020, Alexion announced the launch of the key Phase III research of Ultomiris (ravulizumab) in the treatment of ALS.
Arimoclomol is a small-molecule inducer for the stress response of heat shock, which plays a role in stress cells by stimulating their own heat shock response and can amplify the production of heat shock protein (HSP). HSP can help misfolded proteins return to normal function; or when these proteins can't return to normal functionality, it can also collect the proteins and make them no longer produce toxicant accumulation with the recovery system of the cells - lysosomes, which will help reduce the accumulation of misfolded proteins. And these accumulation of proteins may be the cause of many diseases and symptoms. In July 2019, the Phase III clinical research of arimoclomol in the treatment of ALS completed patients' enrollment ahead of schedule. In addition, the drug has been developed to treat sporadic inclusion body myositis (siIBM) and two lysosomal storage disorders (Niemann-Pick Disease type C [NPC] and Gauchers disease [GD]).
Masitinib is a new type of tyrosine kinase inhibitor administered orally, which targets mast cells and macrophages by inhibiting a limited number of kinases. Based on its unique mechanism of action, masitinib has the potential to be developed for the treatment of many diseases such as tumors, inflammatory diseases and some diseases related to central nervous system.
MN-166 (ibudilast) is the first oral small molecule phosphodiesterase (PDE)-4, phosphodiesterase (PDE)-10 inhibitor and macrophage migration inhibitory factor (MIF) inhibitor, which can inhibit proinflammatory cytokine and promote neurotrophic factors. Ibudilast has been proved to have anti-inflammatory and neuroprotective effects in preclinical and clinical research. In December 2015, MN-166 was granted fast-track qualification for the treatment of ALS by FDA.
CDNF, or Cerebral Dopamine Neurotrophic Factor, is a globally patented neuroprotective and neurotrophic protein owned by Herantis. In the disease model of ALS, CDNF can significantly increase the survival time and reduce symptoms. In 2016, the drug was granted the qualification of orphan drug for the treatment of ALS by EMA.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


