PharmaSources/YiDecember 02, 2021
Tag: Selpercatinib Capsule , NSCLC , RET inhibitors
On November 9, according to the CDE official website, the listing application of Eli Lilly's RET inhibitor "Selpercatinib Capsule" was accepted.
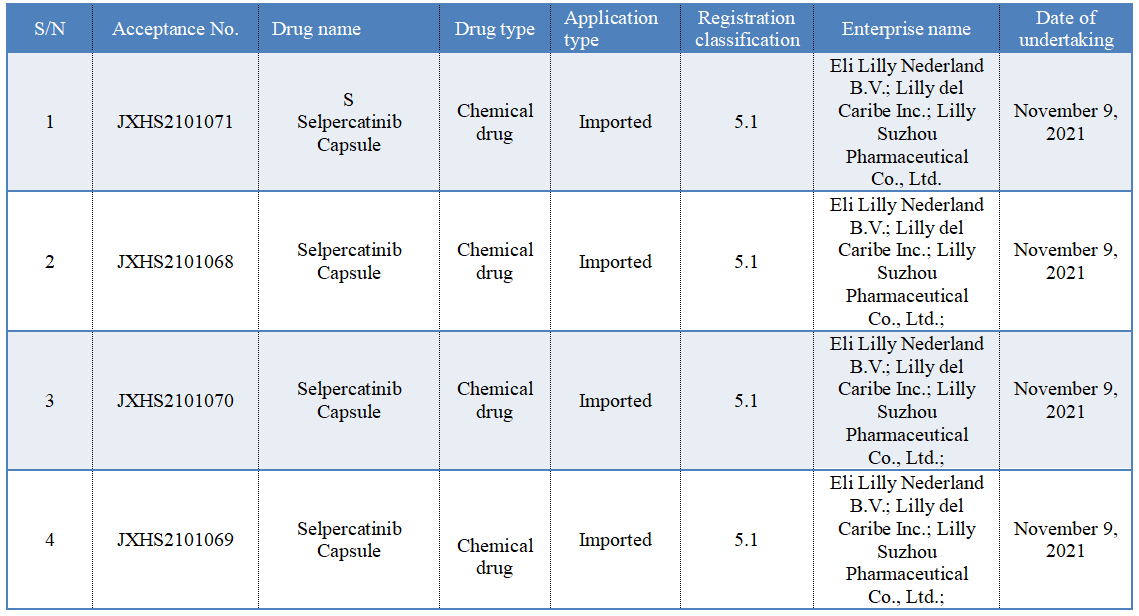
Previously, CDE has given the priority for approval of this drug, and the proposed indications are: (1) Locally advanced or metastatic RET fusion-positive non-small-cell lung cancer (NSCLC); (2) Adults and children aged 12 and above with advanced or metastatic RET-mutant medullary thyroid cancer (MTC) and in need of systematic treatment; and adults and children aged 12 and above with RET fusion-positive advanced or metastatic thyroid carcinoma and in need of systematic treatment and radioactive iodine treatment (if applicable).
Selpercatinib, also known as LOXO-292, is an oral, potent and highly selective inhibitor of RET kinase, which is designed to inhibit native RET signaling as well as anticipated acquired resistance mechanisms. The drug was developed by Loxo Oncology. In 2018, it was granted the breakthrough drug qualification by FDA to treat type 3 patients: (1) After receiving platinum-based chemotherapy and PD-1 or PD-L1 tumor immunotherapy, the metastatic RET fusion-positive NSCLC patients whose condition was worsened and in need systematic treatment (general treatment); (2) Patients with RET-mutant medullary thyroid cancer (MTC) who had gotten worse after previous treatment and have no acceptable alternative treatment options and need systematic treatment; (3) Patients with advanced RET fusion-positive thyroid carcinoma who had gotten worse after receiving other regimens and have no acceptable alternative treatment regimens and need systematic treatment.
In addition, selpercatinib was granted orphan drug qualification by FDA in 2019 for treatment: RET fusion-positive NSCLC, RET fusion-positive and RET-mutant thyroid cancer, including poorly differentiated thyroid cancer, undifferentiated or anaplastic thyroid cancer, MTC, locally advanced or metastatic follicular or papillary thyroid carcinoma.
In January 2019, Eli Lilly reached a final agreement with Loxo Oncology to acquire Loxo Oncology in cash of 235 US dollars (about 8 billion US dollars in total) per share and obtain a variety of drugs including selpercatinib.
In May 2020, selpercatinib (trade name: Retevmo) is approved by FDA for the treatment of adult patients with metastatic RET fusion-positive NSCLC; and adults and children aged 12 and above with advanced or metastatic RET-mutant medullary thyroid cancer in need of systematic treatment; and adults and children aged 12 and above with RET fusion-positive advanced or metastatic thyroid carcinoma and in need of systematic treatment and radioactive iodine treatment (if applicable). According to the company's financial report, the sales volume of the drug reached 36.6 million US dollars in 2020 and 42.5 million US dollars in the first half of 2021.
In China, selpercatinib (LOXO-292) has registered 3 clinical trials, as shown in the following figure. According to the progress of clinical trials, the author speculates that the listing application of selpercatinib is based on the data of foreign clinical trials.
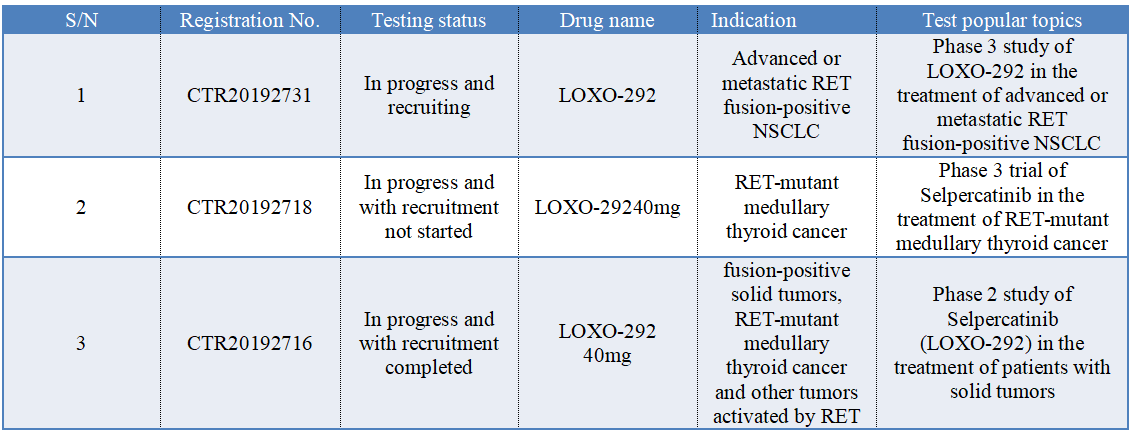
Selpercatinib was approved in the United States based on data from Phase I/II clinical research LIBRETTO-001. This research is the largest clinical trial for patients with RET-driven cancer (N=702), which includes patients who had initial treatment and patients with advanced solid tumor who had previously received some kinds of treatment, including RET fusion-positive NSCLC, RET-mutant MTC, RET fusion-positive thyroid carcinoma and other RET-mutant solid tumors. The main efficacy outcomes were ORR and DoR evaluated by the independent evaluation committee.
Research data published in the New England Journal of Medicine (NEJM) in August 2020 shows that: (1) For RET fusion-positive NSCLC: among untreated patients, 85% of them achieved the result of partial response, and 10% of them were in stable condition. After six months of treatment, 90% of the patients' responses were ongoing. Among the patients who have previously received treatment: 2% of the them got complete response, 62% of partial response, and 29% of stable condition. The median sustained response duration was 17.5 months, and 63% of patients were still in response at the median follow-up time of 12 months. The median progression-free survival (PFS) was 16.5 months. (2) For RET-mutant medullary thyroid carcinoma: in 55 patients with RET-mutant medullary thyroid carcinoma who had received treatment before, the objective response rate (ORR) was 69%, and the one-year progression-free survival rate was 82%; in 88 patients with RET-mutant medullary thyroid carcinoma who hadn't received treatment before, the objective response rate (ORR) was 73%, and the one-year progression-free survival rate was 92%; (3) In 19 treated RET fusion thyroid carcinoma patients, the objective remission rate (ORR) was 79%, and one-year progression-free survival rate was 64%.
RET gene is located on the long arm of chromosome 10 and encodes a receptor tyrosine kinase, which is expressed in normal neurons, sympathetic and parasympathetic ganglia, thyroid C cells, adrenal myeloid cells, urogenital tract cells and testicular germ cells. Activated proteins can activate downstream signaling pathways (including RAS, MAPK, ERK, PI3K, AKT, etc.), which lead to cell proliferation, migration and differentiation.
RET gene mutations, including fusion and point mutations, occur in many tumors, but the incidence of RET mutations varies among different tumors. According to statistics, about 1%-2% of NSCLC patients have RET fusion gene, more than 60% of MTC patients have RET gene point mutation, and 10% of papillary thyroid carcinoma patients have RET gene fusion.
At present, two RET inhibitors have been approved globally (see the table below for details), among which Gavreto was introduced into China by Stone Pharma, and was approved in China in March this year for locally advanced or metastatic RET fusion-positive NSCLC patients who had previously received platinum-based chemotherapy. This time, the listing application of selpercatinib was submitted in China and it is expected to be the second approved RET inhibitor in China. If you want to buy this kind of medical products online, then Pharmasources would be your best choice.
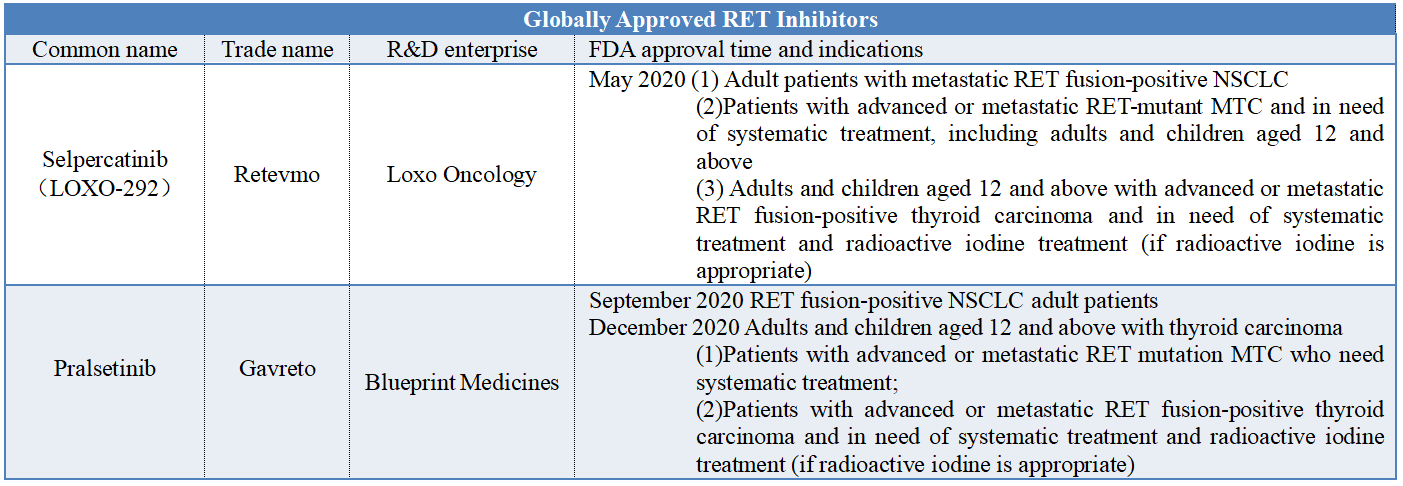
In addition, there are many RET inhibitors under research in China, as shown in the following table. Among them, the progress of KL590586 independently developed by Kelun Biotech is rapid and it has been used to treat advanced solid tumors with RET fusion or mutation. Preclinical study shows that KL590586 has high selectivity to RET kinase and shows good anti-tumor activity and safety in animal models. Compared with similar drugs on the market in the world, it has advantages in animal blood/brain exposure, and is effective for many reported clinical drug-resistant mutations. In addition, this drug has the potential to treat tumor brain metastasis and overcome clinical drug-resistant mutations. In March this year, Kelun Biotech exclusively authorized Ellipses with compensation for the rights of the drug in Europe, America and other regions.

Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


