David Orchard-WebbDecember 02, 2021
Tag: Oncolytic Agent , Imlygic , RIGVIR
Oncolytic therapies, drugs which trigger cancer cell death, lysis, and an immune response, have been regulatory approved for just under two decades, globally. The pipeline is vast, however so far only five drugs in this class of therapies have been approved by regulatory agencies across the world. In this article the approved drugs will be discussed and a few of the most promising candidates in phase III clinical trials.
Table 1: Regulatory approved oncolytic agents.
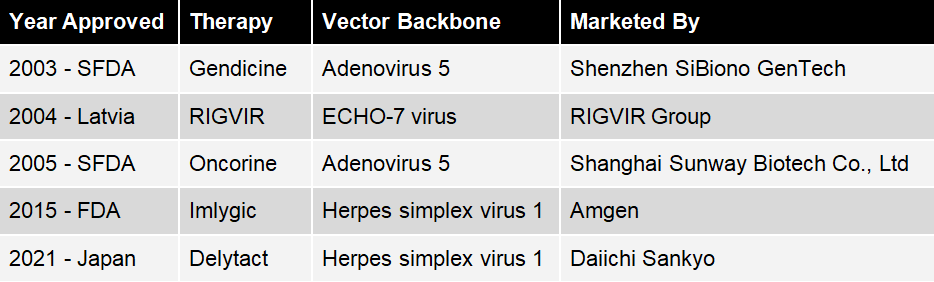
In 2003 Gendicine, produced by Shenzhen SiBiono GenTech, was approved by the State Food and Drug Administration of China (SFDA) for head and neck squamous cell carcinoma (HNSCC) [1]. It is a replication-incompetent, recombinant, serotype 5 human adenovirus (Ad5) engineered to contain the human wild-type p53 tumor-suppressor gene. It was considered a world-first in gene-therapy at the time.
Gencidine has been a clinical and commercial success. In 2018 it was reported that cumulatively a total of 169,571 vials, containing 1.0 x 1012 vector particles each, had been administered to more than 50,000 patients. One injection is given per week for four to eight weeks. Among those patients about 5,000 were international, from over 50 nations outside of China [2]. Over 66 million US$ in sales have been accrued, the cost of each dose reportedly being around US$ 387 [3].
Originally developed in the 1960s, in 2004, RIGVIR was approved by Latvian regulatory authorities and was recently approved in Georgia. Currently manufactured by the RIGVIR Group, it is an oncolytic, non-pathogenic, unmodified ECHO-7 virus, originally adapted for melanoma [4]. On May 31st 2019 the State Agency of Medicines in Latvia suspended the licence for RIGVIR, due to substandard manufacturing resulting in lower than advertised virus titres per vial [5].
In 2005 the SFDA approved a classic (replication competent) oncolytic virotherapy called Oncorine for HNSCC, which was developed by Shanghai Sunway Biotech Co., Ltd [6]. Oncorine is an Ad5 with an E1B and E3 gene deletion. The development in the USA of a very similar virus called Onyx-15 was halted at the outset of a phase III trial due to a funding crisis. This funding crisis led to a ten year lag behind China in the approval of an oncolytic virus.
Oncorine is injected intratumorally, 1-3 vials (5.0 × 1011 ~ 1.5 × 1012 virus particles) per day, combined with 5-FU and cis-pla-tin chemotherapies. It has not been approved in the USA.
In October 2015, the US food and drug administration (FDA) approved Im-ly-gic, for the treatment of melanoma in patients with inoperable tumors [7]. It was developed by BioVex, Inc. and taken to market by Amgen. In Jan 2016 it was approved in Europe for some inoperable melanoma [8]. According to reports it is also available in China [9].
Im-ly-gic is a herpes simplex virus 1 (HSV-1) based oncolytic vector delivered via injection. It was generated from a fresh isolation of HSV-1 virus (JS1) and has a GM-CSF replacement of the two copies of the ICP34.5 gene which normally reverses the interferon induced phosphorylation of the α subunit of the eukaryotic initiation factor 2 (EIF2S1) [10]. The interferon pathway is usually disrupted in cancer thus lending the vector specificity to cancer cells.
It was launched at US$ 65,000 per patient. It is not priced equally in every region. Germany’s Federal Joint Committee (G-BA) published an unfavorable final decision on the early benefit assessment of Im-ly-gic, and the price was halved. That said, the health technology assessment (HTA) in the UK was far more favorable, with NICE recommending the treatment.
In June 2021, Japan’s Ministry of Health, Labour and Welfare (MHLW) granted conditional approval to Daiichi Sankyo’s Delytact, a triple-mutated, replication-conditional HSV-1 for the treatment of malignant glioma [11]. It was jointly developed with the University of Tokyo’s Institute of Medical Science. Daiichi Sankyo is required to conduct post-marketing evaluations, and to collect efficacy and safety data for Delytact until the company files for full marketing approval.
The price will depend on the decision of Japan’s National Health Insurance (NHI) reimbursement plan. The number of glioma cases in Japan is estimated to be around 5,000 annually and the number of malignant glioma cases is estimated to be about 2,800 annually [12].
In September 2021, Genelux Corporation (California, USA), a clinical-stage immunotherapy company, and Newsoara BioPharma Co., Ltd., (Suzhou BioBAY, China) announced an agreement to development and commercialization of Olvi-Vec []. The Olvi-Vec platform is a non-pathogenic oncolytic vaccinia virus, engineered to increase its safety, tumor selectivity and therapeutic efficacy.
Genelux is currently planning a US based Phase 3 clinical trial in platinum resistant/refractory ovarian cancer. Newsoara shall have exclusive rights in Greater China (mainland China, Hong Kong, S.A.R., China, Macau, S.A.R., China and Taiwan, China), collaborate on the development of novel oncolytic immunotherapeutics, and will have the future right to manufacture licensed products.
There are many other oncolytic agents under development including but not limited to those based on HSV, Adenovirus, Vaccinia Virus, Reovirus, Newcastle Disease Virus (NDV) and Vesicular Stomatitis Virus (VSV). If you want to buy medical supplies, then Pharmasources would be your best choice.
The history of oncolytic agent development is a long one, but only in the last twenty years have they gained regulatory approval for marketing. The pace of development has since increased and in June of this year (2021) a new oncolytic therapy, Delytact, was granted conditional approval in Japan. Furthermore, promising platforms such as Olvi-Vec and others seem set to gain approval in the USA and China in the years ahead.
1. Pearson, Sue, Hepeng Jia, and Keiko Kandachi. ‘China Approves First Gene Therapy’. Nature Biotechnology 22, no. 1 (January 2004): 3–4. doi:10.1038/nbt0104-3.
2. Zhang, Wei-Wei, et al. “The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic.” Human Gene Therapy, vol. 29, no. 2, 2018, pp. 160–179., https://doi.org/10.1089/hum.2017.218.
3. “Cancer Drug Divides Opinion.” Financial Times, https://www.ft.com/content/dbb3f2fe-a206-11d9-8483-00000e2511c8.
4. CANCERactive. “Rigvir Virotherapy and Immunotherapy.” CANCERactive, 8 June 2017, https://m.canceractive.com/article/rigvir-virotherapy-and-immunotherapy.
5.De facto. “Rigvir Cancer Treatment at Center of Fresh Controversy.” / Article, LSM, 25 Mar. 2019, https://eng.lsm.lv/article/society/health/rigvir-cancer-treatment-at-center-of-fresh-controversy.a313826/.
6. Garber, Ken. ‘China Approves World’s First Oncolytic Virus Therapy For Cancer Treatment’. Journal of the National Cancer Institute 98, no. 5 (3 January 2006): 298–300. doi:10.1093/jnci/djj111.
7. ‘FDA Approves Amgen’s Injected Immunotherapy for Melanoma’. Reuters, 27 October 2015. http://www.reuters.com/article/us-amgen-fda-idUSKCN0SL2YH20151027.
8. Semedo, Daniela, and PhD. ‘Metastatic Melanoma Therapy, Im-ly-gic, Now Available in EU’. Immuno-Oncology News, 7 January 2016. http://immuno-oncologynews.com/2016/01/07/metastatic-melanoma-therapy-im-ly-gic-now-available-eu/.
9. “Global Gene Therapy Products on the Market - Medical News.” Medical Trend, 4 Mar. 2021, https://medicaltrend.org/2021/03/04/gobal-gene-therapy-products-on-the-market/.
10. Liu BL, Robinson M, Han Z-Q, Branston RH, English C, Reay P, et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther 2003; 10:292–303. https://doi.org/10.1038/sj.gt.3301885.
11. Cairns, Sophie. “Delytact: The World's First Oncolytic Virotherapy for Brain Cancer.” Pharmaceutical Technology, https://www.pharmaceutical-technology.com/pricing-and-market-access/delytact-the-worlds-first-oncolytic-virotherapy-for-brain-canc-html.
12. “Brain Tumor Registry of Japan (2005-2008).” Neurologia Medico-Chirurgica, vol. 57, no. Supplement-1, 2017, pp. 9–102., https://doi.org/10.2176/nmc.sup.2017-0001.
13. Genelux Corporation. “Genelux and Newsoara Announce Collaboration and License Agreement for Oncolytic Immunotherapies.” PR Newswire, 28 Sept. 2021, https://www.prnewswire.com/news-releases/genelux-and-newsoara-announce-collaboration-and-license-agreement-for-oncolytic-immunotherapies-301386251.html.
David Orchard-Webb, Ph.D., is a technical writer with broad interests including health & technology writing, plus extensive training and knowledge of biomedicine and microbiology. My Ph.D. and postdoc were in oncology and developing cancer medicines. I provide technical medical and other writing services for projects ranging from “knowledge automation” to pure pharma, to food safety, to the history of science, and everything in between. I also provide white papers, ebooks, meta-analysis reviews, editing, consulting, business, and market research-related activities in biomedicine, technology, and health. In addition to its well-known role in the development of medicines, I am a big believer in biotechnology’s ability to revolutionize industries such as food-tech, agtech, textiles & fashion.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


