PharmaSources/YiNovember 08, 2021
Tag: pediatric drugs , growth hormone , NMPA
Recently, cyclophosphamide capsules of Hengrui Medicine and human growth hormone recombination injection of GeneScience Pharmaceuticals were proposed to be included in the priority review by CDE because their new varieties, formulation and specifications conform to children's physiological characteristics.
Previously, human growth hormone recombination injection of Anke Biotechnology was also included in the priority review by CDE for pediatric drugs, and it has been approved by NMPA for the treatment of slow growth of children, severe burns and idiopathic short stature caused by endogenous growth hormone deficiency.
There are significant differences between children who are in the growth and development period and adults in the hepatorenal function in terms of drug metabolism and tolerance to adverse reactions. However, the number of approved pediatric drugs in China is limited, which leads to such problems as fewer varieties, formulations, specifications and more adverse reactions in the pediatric drug market.
According to public information, in 2018, the market size of pediatric drugs in China reached about 78.607 billion yuan, with a year-on-year growth of 11.72%. With the release of the second-child and third-child policy, the number of children will continue to increase, so will the market demand for pediatric drugs. The market size is expected to expand.
In recent years, China has paid more and more attention to pediatric drugs, and relevant departments have successively issued a series of policies and guiding principles, involving the R&D, approval, medical products supply and clinical application of pediatric drugs, as shown in the table below. Moreover, Center for Drug Evaluation, NMPA has also opened a column on pediatric drugs, in which policies, regulations and guiding principles and other documents related to pediatric drugs can be centralized publicized. It is convenient for research institutions and enterprises to prepare application materials in advance, so as to improve the adoption efficiency.
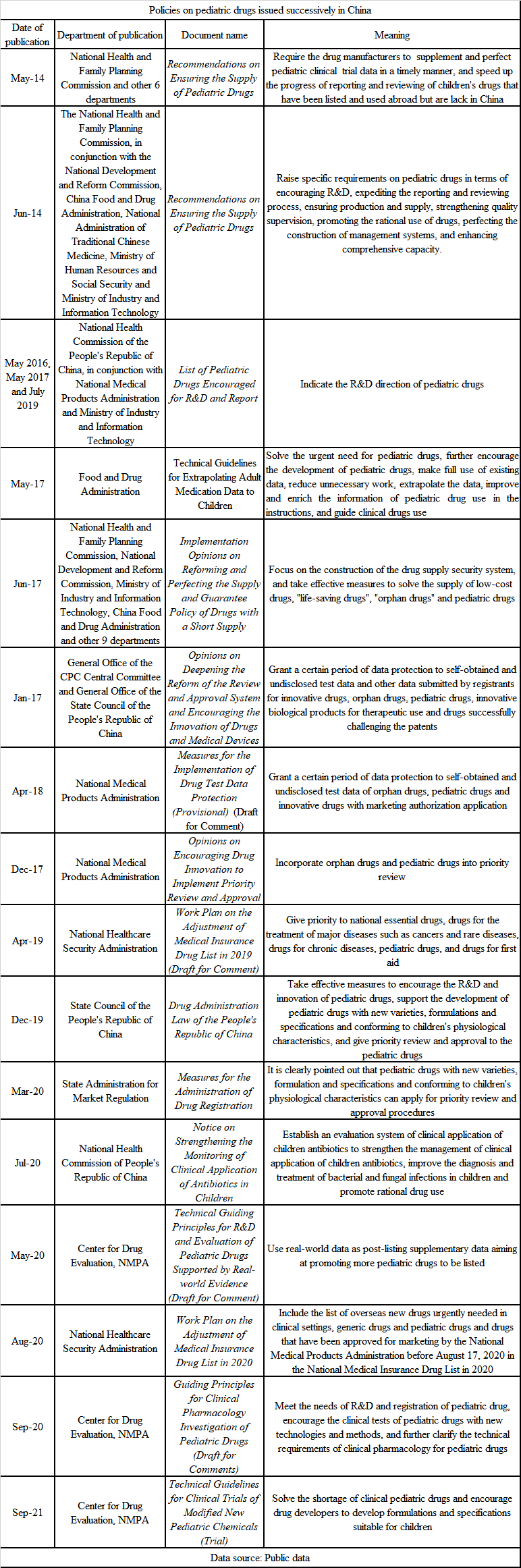
At present, a series of policies and guidance documents issued by China have achieved initial success. It is reported that in 2020, NMPA approved a total of 26 specific drugs and extrapolated drugs for children, with a year-on-year growth of 36.8%, including 17 Chinese drugs (an increase of 46.2%). In 2021, the number of approved varieties of pediatric drugs keeps growing. Up to now, 28 pediatric drugs have been approved for marketing. In addition, 45 specific drugs for children and 21 extrapolated drugs for children indications are under review, among which 22 varieties have been included in the priority review and approval.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


