PharmaSources/YiNovember 01, 2021
Tag: telavancin hydrochloride , vancomycin , glycopeptide antibiotics
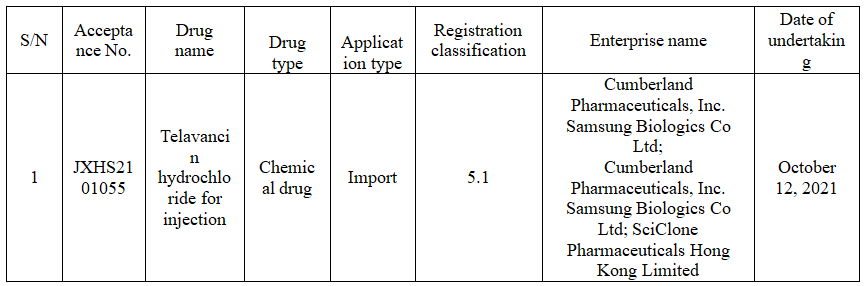
On October 12, CDE official website showed that the import application submitted by SciClone Pharmaceuticals for type 5.1 telavancin hydrochloride for injection was accepted.
Telavancin (Vibativ) is a kind of glycopeptide antibiotics. It is based on the structure of vancomycin, and is obtained by introducing the fatty chain into the amino group of glycosyl through chemical modification, and introducing the phosphate methylamine methyl group into 7th aromatic amino acid. At present, Vibativ has been approved by FDA to treat complicated skin and skin structure infection (cSSSI) of adults caused by gram-positive bacteria (including methicillin-sensitive staphylococcus aureus (MSSA) and methicillin-resistant (MRSA) strains), and adult hospital-acquired and ventilator-associated bacterial pneumonia (HABP/VABP) caused by staphylococcus aureus.
Vibativ was jointly developed by Theravance and astellas and then being sold to Cumberland Pharmaceuticals by Theravance. In 2015, SciClone Pharmaceuticals reached an agreement with Theravance to obtain the exclusive development and commercialization rights of Vibativ in Greater China (including Chinese mainland, Hong Kong, S.A.R., China, Macau, S.A.R., China and Taiwan, China) and Vietnam.
In addition, telavancin has obtained a granted patent CN100469788 of chemical compound in China and it will expire in 2021. At present, Chiatai Tianqing has laid out the generic drug market of telavancin.
Glycopeptide antibiotics, produced by streptomyces or actinomycetes, inhibit the synthesis of bacterial cell walls by combining with the tail of aminoacyl-D alanyl-D alanine of bacterial N-acetyl muramic acid pentapeptide, thus killing the bacterial. Therefore, they are widely used to treat infectious diseases caused by Gram-positive bacteria.
At present, many glycopeptide antibiotics have been approved globally, such as vancomycin, norvancomycin, teicoplanin, dalbavancin, oritavancin and telavancin. Vancomycin, norvancomycin and teicoplanin are the first generation of glycopeptide antibiotics, while dalbavancin, oritavancin and telavancin are the second generation of glycopeptide antibiotics.
Vancomycin is the first glycopeptide antibiotics in the world, which can not only inhibit the synthesis of bacterial cell wall, but also change the permeability of bacterial cell membrane and hinder the synthesis of bacterial RNA. In addition, it has a strong bactericidal effect on gram-positive bacteria such as staphylococcus aureus, staphylococcus epidermidis, streptococcus pyogenes, streptococcus pneumoniae, streptococcus viridans and most of enterococci.
Teicoplanin is a new glycopeptide antibiotics similar to vancomycin. Compared with vancomycin, teicoplanin is easier to penetrate in tissues and cells with more fatty acid side chains, which improves lipophilicity.
Dalbavancin is obtained by introducing 3- (dimethylamino)-1-propylamine into the C-terminal carboxyl of the structure of teicoplanin analogue A40926 by chemical method. It is similar to the active mechanism of natural glycopeptide antibiotics, but its biological activity is obviously enhanced. Dalbavancin (trade name: Dalvance) was originally discovered by Vicuron Pharmaceuticals (later acquired by Pfizer). Later, it was sold to Durata by Pfizer. In May 2014, it was approved by FDA to treat adult patients with acute bacterial skin and skin structural infection (ABSSSI) caused by Gram-positive bacteria (including methicillin-resistant staphylococcus aureus, MRSA), and in July this year, it was approved by FDA to treat pediatric patients with ABSSSI caused by Gram-positive bacteria (including MRSA). It is reported that the global sales of Dalvance reached 87.9 million US dollars in 2019.
Oritavancin is a structural derivative modified with 4'-chlorobiphenyl methyl on the amino group of 4-amino acid disaccharide of chloroorient toxin A. The introduction of modified side chain groups on its glycosyl promotes the anchoring with the cell membrane of targeted strains and enhances its antibacterial activity. The drug has a long half-life and good activity in inhibiting VanA enterococcus (vancomycin-tolerant). It is also effective in treating infections of skin and soft tissue, and characterized by safety and well toleration. The original oritavancin (trade name: Orbactiv®) was developed by Medicines Co., and was approved by FDA in August 2014 to treat adult patients with ABSSSIs caused by sensitive Gram-positive bacteria.
In China, vancomycin, norvancomycin and teicoplanin have been approved, but the second-generation glycopeptide antibiotics dalbavancin, oritavancin and telavancin have not been approved. Telavancin introduced by SciClone Pharmaceuticals is the first second-generation glycopeptide antibiotics applied for production in China.
Infectious disease is a serious disease that seriously endangers human health, and it is one of the causes of death and inducing serious complications, such as multiple organ failure. According to the report of WHO, the amount of people died of infectious diseases accounts for 25% of that of all diseases, and the drug resistance of pathogenic bacteria causing infection has become a severe challenge facing the whole world.
Developing new antibiotics is the main strategy of WHO to deal with drug-resistant bacterial infections. Antibiotics companies have done a lot to new antibiotics. In recent years, many new antibiotics have been approved worldwide, as shown in the following table.
Among them, ceftazidime avibactam sodium is a new enzyme inhibitor compound preparation, which is composed of the third-generation cephalosporin antibiotic ceftazidime and the sodium salt of new β-lactamase inhibitor avibactam. It can treat infections caused by drug-resistant gram-negative bacteria, including CRE, multi-drug-resistant pseudomonas aeruginosa and extended-spectrum lactamase (ESBLs) bacteria. The ceftazidime avibactam sodium was originally developed by Pfizer and approved in China in 2019. Recently, Qilu Pharmaceutical has obtained the first generic drug production approval of this drug.
Tedizolid and contezolid are oxazolidinone antibiotics. Among them, terzolamide was originally developed by Dong-A Pharmaceutical Co., Ltd. of Korea, and then the company authorized Trius Therapeutics, Cubist and Bayer for commercial development. In 2019, it was approved in China to treat adult acute skin and skin tissue infection caused by Gram-positive bacteria. At present, 8 Chinese companies have submitted their listing application for generic drug. Contezolid is independently developed by MicuRx Pharmaceuticals, and was approved in China in June this year to treat infections caused by drug-resistant bacteria, such as methicillin-resistant staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE).
Eravacycline and omadacycline are new tetracycline antibiotics, and the listing application of both of them has been submitted in China. Among them, eravacycline was approved by FDA in August 2018 to treat adult complicated abdominal infection, and then it was introduced into China by Everest Medicines from Tetraphse Pharmaceutical. Omacycline is a new type of 9-aminomethyl cycline antibiotic, which is a semi-synthetic derivative of minocycline. It was approved by FDA in October 2018 to treat adult patients with CABP and ABSSSI. In 2017, Zai Lab introduced the drug from Paratek Pharmaceuticals.
Yi, a pharmacist pays attention to the research and development trends of new drugs at home and abroad, expects to improve himself in the continuous input and output, and grow together with medical we-media.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


