PharmaSources/Ye FenghongNovember 01, 2021
Tag: BET inhibitor , BET protein , Betta Pharmaceuticals , malignant hematological tumors
Recently, CDE official website showed that the clinical trial application of Betta Pharmaceuticals for BPI-23314 tablets, a type 1 new drug, has been given the implied license. This new drug is used to treat malignant hematological tumors (including but not limited to myeloproliferative tumors, such as myelofibrosis and myelodysplastic syndrome. This is the third clinical trial license for the drug.
According to the announcement issued by Betta Pharmaceuticals, BPI-23314 is a new molecular chemical entity independently developed by it. It is a new, powerful and selective oral small molecule inhibitor of bromine structural domain and extra terminal domain (BET) protein family, which has a brand-new mechanism of degrading target protein and is intended to be used for the treatment of hematologic tumor, breast cancer and lung cancer.
Upon inquiry, drugs with the same target at home and abroad are in the clinical trial stage, and haven't yet been listed.
At present, BET family proteins have become one of the new targets for scientists to develop innovative anti-cancer therapies. BET protein family consists of BRD2, BRD3, BRD4 and BRDT. Studies have shown that BET protein is widely expressed in human tissues and regulate gene transcription. Among them, bromine structural domain protein 4 (BRD4) is the most important functional protein in BET family, which can regulate DNA replication and gene expression through histone acetylation, thus affecting the cell cycle procession. In recent years, it has been found that the dysfunction of BET family is also closely related to the occurrence, invasion and metastasis of tumors.
As a new anti-cancer drug, BET inhibitor is promising in treating blood cancers, such as leukemia and lymphoma. BET inhibitors can inhibit growth of tumor by blocking the function of BET protein. R&D of BET protein selective inhibitors is a new target at present. At present, many pharmaceutical companies and hospital supply companies have engaged in the R&D of this selective inhibitor.
NHWD-870 is a novel and powerful BET inhibitor, which is intended to be developed for the treatment of various solid tumors. This is the first innovative antitumor drug of Wenda Pharma entering into the clinical development stage.
NHWD-870 can inhibit BRD4 structural domain by targeting, and induce apoptosis of tumor cells, thus achieving anti-tumor effect. According to public data, the drug was initially developed by Professor Chen Xiang and Professor Yin Mingzhu from the R&D team of Xiangya Hospital Central South University. And now, the subsequent R&D is carried out by Wenda Pharma and Hengya Pharm.
Researchers found that NHWD-870 can not only down-regulate c-MYC and directly inhibit the proliferation of tumor cells, but also block the proliferation of tumor-related macrophages through a variety of mechanisms, some of which are to stimulate the expression and secretion of factor CSF1 by reducing the macrophage colony of tumor cells.
Preclinical studies indicate that NHWD-870 shows strong anti-tumor activity in nine different mouse tumor models. Moreover, the inhibitory activity of NHWD-870 is 5-50 times higher than that of similar compounds. According to the result of pharmacokinetic study, oral NHWD-870 shows good tumor penetration.
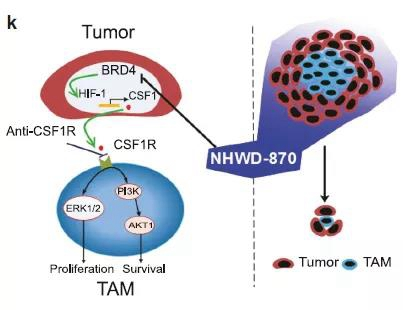
Mechanism of NHWD-870 (Source: Reference 1)
JAB-8263 is a BET inhibitor of Jacobio. JAB-8263 is intended to be used in the treatment of various cancer types associated with increased MYC expression, including various solid tumors and blood cancers. According to the preclinical studies, JAB-8263 shows good anti-tumor effect in various solid tumors and hematologic tumors, and has higher activity than similar drugs. JAB-8263 has obtained the IND approval for the treatment of solid tumors in China and the United States. In July 2020, it was approved for IND by FDA for the treatment of solid tumor patients, and the first patient was enrolled in the United States in November of the same year. In September 2020, CDE official website showed that the declaration of JAB-8263 tablets, a BET inhibitor by Jacobio, weas accepted, and the new drug was intended to be used for the treatment of solid tumors.
HH3806 of Haihe Pharmaceutical is a BD2 selective BET inhibitor. The inhibitory activity of the inhibitor on BD2 structural domain is 200 times more than that of BD1. In animal experiments of preclinical research, compared with non-selective BET inhibitors, HH3806 retains good efficacy against some solid tumors and hematologic tumors, but its harm to normal cells is greatly reduced, and its toxicity to the digestive tract and hematology is significantly reduced. This result indicates that the tolerated dose of HH3806 may be higher and the safety window of efficacy may be larger in clinical research.
HH3806 project is still in the preparation stage of preclinical IND, and many tests targeting HH3806 project are being carried out, including a full set of GLP toxicological tests and pharmacodynamic tests. It is expected that IND applications will be submitted to FDA in the United States and CDE in China at the end of 2021.
To sum up, the competition of BET targets is still quite fierce. But because the research of BET targets is still in an early clinical stage, it is still too early to draw a conclusion whether this small molecule drug target can be finally made into a medicine, and more clinical data are needed.
1.Yin, M., Guo, Y.,et al. (2020). Potent BRD4 inhibitor suppresses cancercell-macrophage interaction. Nature Communications.doi:10.1038/s41467-020-15290-0?;
2.Tenti E, Papayannidis C, Marconi G, et al. Efficacy of azacitidine in the treatment of adult patients aged 65 years or older with AML[J]. Expert Opin Pharmacother, 2016, 17(18): 2479-2486.?DOI:10.1080/14656566.2016.1258056;
3.Martin P, Bartlett NL, Rivera IIR, et al. A phaseⅠ, open label, multicenter trial of oral azacitidine (CC-486) Plus R-CHOP in patients with high-risk, previously untreated diffuse large B-cell lymphoma, Grade 3B follicular lymphoma, or transformed lymphoma[J].?Blood, 2017, 130(Supple 1): 192-192.
Ye Fenghong, a medical editor specializing in oncology at a healthcare internet company, has conducted in-depth research on the pathogenesis and clinical treatment of lung cancer and breast cancer. She has previously been involved in the design and synthesis of anti-tumor drugs and has some experience in computer-aided drug design. She is currently devoted to introducing cutting-edge cancer treatment drugs to a wide range of readers, aiming to help more people avoid cancer pain and embrace good health.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


